Ibudilast
Ibudilast(IBD) (development codes: AV-411 or MN-166) is an anti-inflammatory drug used mainly in Japan, which acts as a phosphodiesterase inhibitor, inhibiting the PDE4 subtype to the greatest extent,[1] but also showing significant inhibition of other PDE subtypes.[2][3]
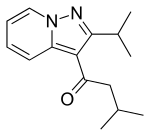 | |
| Clinical data | |
|---|---|
| Trade names | Ketas, Pinatos, Eyevinal |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth (capsules), topical (ophthalmic solution) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.881 |
| Chemical and physical data | |
| Formula | C14H18N2O |
| Molar mass | 230.31 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Medical uses
In Japan, ibudilast oral capsules are approved for the treatment of asthma, and for improvement of dizziness secondary to chronic cerebral circulation impairment associated with sequelae of cerebral infarction.[4] Ibudilast ophthalmic solution is indicated for the treatment of allergic conjunctivitis and hay fever.[5]
It may have some use reducing methamphetamine[6] and alcohol[7] addiction.
Pharmacology
Ibudilast has bronchodilator, vasodilator[8] and neuroprotective effects,[9][10] and is mainly used in the treatment of asthma and stroke.[11] It inhibits platelet aggregation,[12] and may also be useful in the treatment of multiple sclerosis.[13]
Ibudilast crosses the blood–brain barrier and suppresses glial cell activation. This activity has been shown to make ibudilast useful in the treatment of neuropathic pain and it not only enhances analgesia produced by opioid drugs, but also reduces the development of tolerance.[14]
Pharmacodynamics
Ibudilast is principally a PDE4 inhibitor but has also been shown to act as an antagonist at the toll-like receptor 4 (TLR4).[15] This likely plays a large part in its effect, specifically its synergy with opioid drugs, its anti-inflammatory effect, and its own painkilling effect.[16] It is unknown if the PDE4-inhibiting properties potentiate the effects of TLR4 inactivation and/or vice versa, despite that some of their effects are shared, such as inflammation reducing properties.[17] TLR4 antagonists theoretically reverse the increase in pain and inflammation caused by most TLR4 agonists, which include many opiate and opioid drugs.[18]
Clinical trials
References
- Huang, Z; Liu, S; Zhang, L; Salem, M; Greig, GM; Chan, CC; Natsumeda, Y; Noguchi, K (May 2006). "Preferential inhibition of human phosphodiesterase 4 by ibudilast". Life Sciences. 78 (23): 2663–8. doi:10.1016/j.lfs.2005.10.026. PMID 16313925.
- Suzumura, A; Ito, A; Yoshikawa, M; Sawada, M (Aug 1999). "Ibudilast suppresses TNFalpha production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS". Brain Research. 837 (1–2): 203–12. doi:10.1016/s0006-8993(99)01666-2. PMID 10434004.
- Gibson, Lucien C.D.; Hastings, Stuart F.; McPhee, Ian; Clayton, Robert A.; Darroch, Claire E.; MacKenzie, Alison; MacKenzie, Fiona L.; Nagasawa, Michiaki; Stevens, Patricia A.; MacKenzie, Simon J. (2006). "The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family". European Journal of Pharmacology. 538 (1–3): 39–42. doi:10.1016/j.ejphar.2006.02.053. PMID 16674936.
- "Ketas (ibudilast) Capsules 10 mg. Prescribing Information" (PDF). Kyorin Pharmaceutical Co., Ltd. Retrieved 3 October 2016.
- "Ketas (ibudilast) Eye Drops 0.01% Kisuri-no-Shiori (Drug Information Sheet)". Senju Pharmaceutical Co., Ltd. Retrieved 3 October 2016.
- "Cure for Meth Addiction?". 2013-04-03.
- Bell, Richard L. (2015). "Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence". Addiction Biology. 20 (1): 38–42. doi:10.1111/adb.12106. PMC 4017009. PMID 24215262.
- Kishi, Y; Ohta, S; Kasuya, N; Sakita, S; Ashikaga, T; Isobe, M (Fall 2001). "Ibudilast: a non-selective PDE inhibitor with multiple actions on blood cells and the vascular wall". Cardiovascular Drug Reviews. 19 (3): 215–25. doi:10.1111/j.1527-3466.2001.tb00066.x. PMID 11607039.
- Mizuno, Tetsuya; Kurotani, Tohru; Komatsu, Yukio; Kawanokuchi, Jun; Kato, Hideki; Mitsuma, Norimasa; Suzumura, Akio (2004). "Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia". Neuropharmacology. 46 (3): 404–411. doi:10.1016/j.neuropharm.2003.09.009. PMID 14975696.
- Yoshioka, M; Suda, N; Mori, K; Ueno, K; Itoh, Y; Togashi, H; Matsumoto, M (Apr 2002). "Effects of ibudilast on hippocampal long-term potentiation and passive avoidance responses in rats with transient cerebral ischemia". Pharmacological Research. 45 (4): 305–11. doi:10.1006/phrs.2002.0949. PMID 12030794.
- Wakita, H; Tomimoto, H; Akiguchi, I; Lin, JX; Ihara, M; Ohtani, R; Shibata, M (Nov 2003). "Ibudilast, a phosphodiesterase inhibitor, protects against white matter damage under chronic cerebral hypoperfusion in the rat". Brain Research. 992 (1): 53–9. doi:10.1016/j.brainres.2003.08.028. PMID 14604772.
- Rile, G; Yatomi, Y; Qi, R; Satoh, K; Ozaki, Y (May 2001). "Potentiation of ibudilast inhibition of platelet aggregation in the presence of endothelial cells". Thrombosis Research. 102 (3): 239–46. doi:10.1016/s0049-3848(01)00258-4. PMID 11369417.
- Feng, J; Misu, T; Fujihara, K; Sakoda, S; Nakatsuji, Y; Fukaura, H; Kikuchi, S; Tashiro, K; Suzumura, A; Ishii, N; Sugamura, K; Nakashima, I; Itoyama, Y (Oct 2004). "Ibudilast, a nonselective phosphodiesterase inhibitor, regulates Th1/Th2 balance and NKT cell subset in multiple sclerosis". Multiple Sclerosis. 10 (5): 494–8. doi:10.1191/1352458504ms1070oa. PMID 15471363.
- Ledeboer, A; Hutchinson, MR; Watkins, LR; Johnson, KW (Jul 2007). "Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes". Expert Opinion on Investigational Drugs. 16 (7): 935–50. doi:10.1517/13543784.16.7.935. PMID 17594181.
- Jia, Ze-jun; Wu, Fei-xiang; Huang, Qing-hai; Liu, Jian-min (2012-04-01). "[Toll-like receptor 4: the potential therapeutic target for neuropathic pain]". Zhongguo Yi Xue Ke Xue Yuan Xue Bao. Acta Academiae Medicinae Sinicae. 34 (2): 168–173. doi:10.3881/j.issn.1000-503X.2012.02.013. ISSN 1000-503X. PMID 22776604.
- Hutchinson, Mark R.; Zhang, Yingning; Shridhar, Mitesh; Evans, John H.; Buchanan, Madison M.; Zhao, Tina X.; Slivka, Peter F.; Coats, Benjamen D.; Rezvani, Niloofar (2010-01-01). "Evidence that opioids may have toll like receptor 4 and MD-2 effects". Brain, Behavior, and Immunity. 24 (1): 83–95. doi:10.1016/j.bbi.2009.08.004. ISSN 0889-1591. PMC 2788078. PMID 19679181.
- Jin, S.-L. Catherine; Ding, Shiau-Li; Lin, Shih-Chang (2012-06-01). "Phosphodiesterase 4 and its inhibitors in inflammatory diseases". Chang Gung Medical Journal. 35 (3): 197–210. doi:10.4103/2319-4170.106152. ISSN 2309-835X. PMID 22735051.
- Komatsu, Takaaki; Sakurada, Shinobu; Katsuyama, Sou; Sanai, Kengo; Sakurada, Tsukasa (2009-01-01). "Mechanism of allodynia evoked by intrathecal morphine-3-glucuronide in mice". International Review of Neurobiology. 85: 207–219. doi:10.1016/S0074-7742(09)85016-2. ISSN 0074-7742. PMID 19607972.