RPL-554
RPL-554 (LS-193,855) is a drug candidate for respiratory diseases. It is an analog of trequinsin and, like trequinsin, is a highly selective inhibitor of the phosphodiesterase enzyme, PDE3; indeed, it is >3000-times more potent against PDE3 than PDE4.[1] As of October 2015, inhaled RPL-554 delivered via a nebulizer was in development for COPD and had been studied in asthma.[2]
 | |
| Clinical data | |
|---|---|
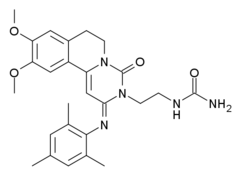
| Other names | 9,10-Dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one |
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| ECHA InfoCard | 100.245.423 |
| Chemical and physical data | |
| Formula | C26H31N5O4 |
| Molar mass | 477.554 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
PDE3 inhibitors act as bronchodilators, while PDE4 inhibitors have an anti-inflammatory effect.[1][3]
RPL554 was part of a family of compounds invented by Sir David Jack, former head of R&D for GlaxoSmithKline, and Alexander Oxford, a medicinal chemist; the patents on their work were assigned to Vernalis plc.[4][5][6]:19-20
In 2005, Rhinopharma Ltd, acquired the rights to the intellectual property from Vernalis.[6]:19-20 Rhinopharma was a startup founded in Vancouver, Canada in 2004 by Michael Walker, Clive Page, and David Saint, to discover and develop drugs for chronic respiratory diseases,[6]:16 and intended to develop RPL-554, delivered with an inhaler, first for allergic rhinitis, then asthma, then for COPD.[6]:16-17 RPL554 was synthesized at Tocris, a contract research organization, under the supervision of Oxford, and was studied in collaboration with Page's lab at King’s College, London.[1] In 2006 Rhinopharma recapitalized and was renamed Verona Pharma plc.[6]
References
- Boswell-Smith V, Spina D, Oxford AW, Comer MB, Seeds EA, Page CP (August 2006). "The pharmacology of two novel long-acting phosphodiesterase 3/4 inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido[6,1-a]isoquinolin-4-one]". The Journal of Pharmacology and Experimental Therapeutics. 318 (2): 840–8. doi:10.1124/jpet.105.099192. PMID 16682455.
- Taylor NP (1 October 2015). "Verona sets sights on PhIIb after COPD drug comes through early trial". FierceBiotech.
- Turner MJ, Matthes E, Billet A, Ferguson AJ, Thomas DY, Randell SH, et al. (January 2016). "The dual phosphodiesterase 3 and 4 inhibitor RPL554 stimulates CFTR and ciliary beating in primary cultures of bronchial epithelia". American Journal of Physiology. Lung Cellular and Molecular Physiology. 310 (1): L59–70. doi:10.1152/ajplung.00324.2015. PMID 26545902.
- US 20040171828, "Derivatives of pyrimido[6,1-a]isoquinolin-4-one", assigned to Verona Pharma PLC
- Boswell-Smith V, Spina D, Oxford AW, Comer MB, Seeds EA, Page CP (August 2006). "The pharmacology of two novel long-acting phosphodiesterase 3/4 inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido[6,1-a]isoquinolin-4-one]". The Journal of Pharmacology and Experimental Therapeutics. 318 (2): 840–8. doi:10.1124/jpet.105.099192. PMID 16682455.
- "Proposed Acquisition of Rhinopharma" (PDF). ISIS Resources, PLC. 23 August 2006.