Crisaborole
Crisaborole, sold under the brand name Eucrisa, is a nonsteroidal topical medication used for the treatment of mild-to-moderate atopic dermatitis (eczema) in people two years or older.[1][2] It was approved by U.S. Food and Drug Administration on Dec 14, 2016[3] and June 6, 2018 by Health Canada[4].
 | |
| Clinical data | |
|---|---|
| Trade names | Eucrisa, /juːˈkrɪsə/ yoo-KRIS-ə |
| Routes of administration | Topical (ointment) |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.225.309 |
| Chemical and physical data | |
| Formula | C14H10BNO3 |
| Molar mass | 251.045 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Side effects
At the site of application, crisaborole may cause burning or stinging. Rarely, there may be an allergic reaction.[5]
Pharmacology
Pharmacodynamics
Crisaborole is a phosphodiesterase-4 inhibitor, mainly acting on phosphodiesterase 4B (PDE4B), which causes inflammation.[6] Chemically, crisaborole is a phenoxybenzoxaborole.[6] Inhibition of PDE4B appears to suppress the release of tumor necrosis factor alpha (TNFα), interleukin-12 (IL-12), IL-23 and other cytokines, proteins believed to be involved in the immune response and inflammation.[6]
Chemistry
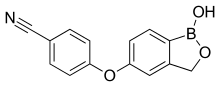
Crisaborole (chemical name: 4-[(1-hydroxy-1,3-dihydro-2,1-benzoxaborol-5-yl)oxy]benzonitrile) is a member of the class of benzoxaboroles characterized by the presence of a boronic acid hemiester with a phenolic ether and a nitrile.[7] Crisaborole crystallizes into two polymorphs that differ in the conformation of the oxaborole ring. A cocrystal with 4,4'-bipyridine has been prepared and studied by X-ray crystallography.[8]
History
Crisaborole was developed by Anacor Pharmaceuticals for the topical treatment of psoriasis.[9][6][10] During preclinical and clinical development, crisaborole was called AN2728 and PF-06930164.[11]
See also
- Tavaborole – a structurally related topical antifungal developed by Anacor
References
- "FDA Approves Eucrisa for Eczema". U.S. Food and Drug Administration. 14 December 2016.
- "Eucrisa (crisaborole) Ointment, 2%, for Topical Use. Full Prescribing Information". Anacor Pharmaceuticals, Inc. Palo Alto, CA 94303 USA. Retrieved 17 December 2016.
- "FDA approves Eucrisa for eczema" (Press release). U.S. Food and Drug Administration. December 14, 2016.
- Government of Canada. "Regulatory Decision Summary - Eucrisa - Health Canada". Health Canada. Archived from the original on 2019-09-14. Retrieved 2019-09-14.
- "PRODUCT MONOGRAPH" (PDF). Government of Canada. 2018-06-07. Archived (PDF) from the original on 2019-04-07. Retrieved 2019-04-07.
- Moustafa, F; Feldman, SR (16 May 2014). "A Review of Phosphodiesterase-Inhibition and the Potential Role for Phosphodiesterase 4-Inhibitors in Clinical Dermatology" (PDF). Dermatology Online Journal. 20 (5): 22608. PMID 24852768.
- "WHO Drug Information, Vol. 29, No. 3, 2015. International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 74" (PDF). World Health Information. p. 391. Retrieved 26 April 2016.
- Campillo-Alvarado, Gonzalo; Didden, Tighe D.; Oburn, Shalisa M.; Swenson, Dale C.; MacGillivray, Leonard R. (2018). "Exploration of Solid Forms of Crisaborole: Crystal Engineering Identifies Polymorphism in Commercial Sources and Facilitates Cocrystal Formation". Crystal Growth & Design. 18 (8): 4416–4419. doi:10.1021/acs.cgd.8b00375. ISSN 1528-7483.
- Nazarian, R; Weinberg, JM (November 2009). "AN-2728, a PDE4 Inhibitor for the Potential Topical Treatment of Psoriasis and Atopic Dermatitis". Current Opinion in Investigational Drugs. 10 (11): 1236–42. PMID 19876791.
- Spreitzer, H (16 August 2016). "Neue Wirkstoffe: Crisaborol". Österreichische Apotheker-Zeitung (in German) (17/2016).
- "Crisaborole". AdisInsight. Retrieved 24 July 2017.