Milrinone
Milrinone, commonly known and marketed under the brand name Primacor, is a medication used in patients who have heart failure. It is a phosphodiesterase 3 inhibitor that works to increase the heart's contractility and decrease pulmonary vascular resistance. Milrinone also works to vasodilate which helps alleviate increased pressures (afterload) on the heart, thus improving its pumping action. While it has been used in people with heart failure for many years, studies suggest that milrinone may exhibit some negative side effects that have caused some debate about its use clinically.[1][2]
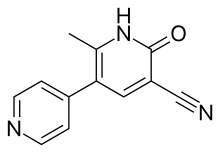 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601020 |
| Pregnancy category |
|
| Routes of administration | IV only |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (as IV bolus, infusion) |
| Protein binding | 70 to 80% |
| Metabolism | Hepatic (12%) |
| Elimination half-life | 2.3 hours (mean, in CHF) |
| Excretion | Urine (85% as unchanged drug) within 24 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.071.709 |
| Chemical and physical data | |
| Formula | C12H9N3O |
| Molar mass | 211.219 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.344 g/cm3 |
| Melting point | 315 °C (599 °F) |
SMILES
| |
InChI
| |
| (verify) | |
Overall, milrinone supports ventricular functioning of the heart by decreasing the degradation of cyclic adenosine monophosphate (cAMP) and thus increasing phosphorylation levels of many components in the heart that contribute to contractility and heart rate. Milrinone use following cardiac surgery has been under some debate because of the potential increase risk of postoperative atrial arrhythmias.[3] However, in the short term milrinone has been deemed beneficial to those experiencing heart failure and an effective therapy to maintain heart function following cardiac surgeries. There is no evidence of any long term beneficial effects on survival.[4] In critically ill patients with evidence of cardiac dysfunction there is limited good quality evidence to recommend its use.[5]
Contractility in the heart
People experiencing some forms of heart failure have a significant decrease in the contractile ability of muscle cells in the heart (cardiomyocytes). This impaired contractility occurs through a number of mechanisms. Some of the main problems associated with decreased contractility in those with heart failure are issues arising from imbalances in the concentration of calcium. Calcium permits myosin and actin to interact which allows initiation of contraction within the cardiomyocytes. In those with heart failure there may be a decreased amount of calcium within the cardiomyocytes reducing the available calcium to initiate contraction. When contractility is decreased the amount of blood being pumped out of the heart into circulation is decreased as well. This reduction in cardiac output can cause many systemic implications such as fatigue, syncope and other issues associated with decreased blood flow to peripheral tissues.
Mechanism of action
cAMP causes increased activation of protein kinase A (PKA). PKA is an enzyme that phosphorylates many elements of the contractile machinery within the heart cell. In the short term this leads to an increased force of contraction. Phosphodiesterases are enzymes responsible for the breakdown of cAMP. Therefore, when phosphodiesterases lower the level of cAMP in the cell they also lower the active fraction of PKA within the cell and reduce the force of contraction.
Milrinone is a phosphodiesterase-3 inhibitor. This drug inhibits the action of phosphodiesterase-3 and thus prevents degradation of cAMP. With increased cAMP levels there is an increase in the activation of PKA. This PKA will phosphorylate many components of the cardiomyocyte such as calcium channels and components of the myofilaments. Phosphorylation of calcium channels permits an increase in calcium influx into the cell. This increase in calcium influx results in increased contractility. PKA also phosphorylates potassium channels promoting their action. Potassium channels are responsible for repolarization of the cardiomyocytes therefore increasing the rate at which cells can depolarize and generate contraction. PKA also phosphorylates components on myofilaments allowing actin and myosin to interact more easily and thus increasing contractility and the inotropic state of the heart. Milrinone allows stimulation of cardiac function independently of β-adrenergic receptors which appear to be down-regulated in those with heart failure.
Clinical use
Milrinone is a commonly used therapy for severe pulmonary arterial hypertension (PAH),[6] often in combination with other medications such as sildenafil.[7]
Adverse effects
Common adverse effects include ventricular arrhythmias (including ventricular ectopy and nonsustained ventricular tachycardia), supraventricular arrhythmias, hypotension, and headache.[8]
References
- Packer, M. (1990). "Calcium channel blockers in chronic heart failure. The risks of "physiologically rational" therapy". Circulation. 82 (6): 2254–2257. doi:10.1161/01.cir.82.6.2254. PMID 2242549.
- Packer, Milton; Carver, Joseph R.; Rodeheffer, Richard J.; Ivanhoe, Russell J.; Dibianco, Robert; Zeldis, Steven M.; Hendrix, Grady H.; Bommer, William J.; Elkayam, Uri; Kukin, Marrick L.; Mallis, George I.; Sollano, Josephine A.; Shannon, James; Tandon, P.K.; Demets, David L. (1991). "Effect of Oral Milrinone on Mortality in Severe Chronic Heart Failure". New England Journal of Medicine. 325 (21): 1468–1475. doi:10.1056/NEJM199111213252103. PMID 1944425.
- Fleming, Gregory A.; Murray, Katherine T.; Yu, Chang; Byrne, John G.; Greelish, James P.; Petracek, Michael R.; Hoff, Steven J.; Ball, Stephen K.; Brown, Nancy J.; Pretorius, Mias (2008). "Milrinone Use is Associated with Postoperative Atrial Fibrillation After Cardiac Surgery". Circulation. 118 (16): 1619–1625. doi:10.1161/CIRCULATIONAHA.108.790162. PMC 2770257. PMID 18824641.
- British National Formulary. 66 ed. London: BMJ Group and Pharmaceutical Press; Sept 2013
- Koster, Geert; Bekema, Hanneke J.; Wetterslev, Jørn; Gluud, Christian; Keus, Frederik; Van Der Horst, Iwan C. C. (2016). "Milrinone for cardiac dysfunction in critically ill adult patients: A systematic review of randomised clinical trials with meta-analysis and trial sequential analysis". Intensive Care Medicine. 42 (9): 1322–1335. doi:10.1007/s00134-016-4449-6. PMID 27448246.
- McNamara, Patrick J.; Shivananda, Sandesh P.; Sahni, Mohit; Freeman, David; Taddio, Anna (January 2013). "Pharmacology of Milrinone in Neonates With Persistent Pulmonary Hypertension of the Newborn and Suboptimal Response to Inhaled Nitric Oxide*". Pediatric Critical Care Medicine. 14 (1): 74–84. doi:10.1097/PCC.0b013e31824ea2cd. ISSN 1529-7535. PMID 23132395.
- Hui-li, Gan (June 2011). "The Management of Acute Pulmonary Arterial Hypertension: The Management of Acute Pulmonary Hypertension". Cardiovascular Therapeutics. 29 (3): 153–175. doi:10.1111/j.1755-5922.2009.00095.x. PMID 20560976.
- "Milrinone Lactate Monograph". drugs.com.