Aildenafil
Aildenafil (methisosildenafil) is a synthetic chemical compound that is a structural analog of sildenafil (Viagra).[1] It was first reported in 2003,[2] and it is not approved by any health regulation agency. Like sildenafil, aildenafil is a phosphodiesterase type 5 inhibitor.
 | |
| Names | |
|---|---|
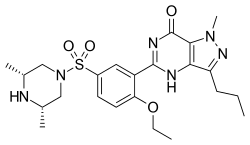
| IUPAC name
5-(5-(((3R,5S)-3,5-Dimethylpiperazin-1-yl)sulfonyl)-2-ethoxyphenyl)-1-methyl-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-7(4H)-one | |
| Other names
Methisosildenafil | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C23H32N6O4S |
| Molar mass | 488.61 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aildenafil has been found as an adulterant in a variety of supplements which are sold as "natural" or "herbal" sexual enhancement products.[3][4][5][6][7] The United States Food and Drug Administration has warned consumers that any sexual enhancement product that claims to work as well as prescription products is likely to contain such a contaminant.[8]
References
- Zhao, Xia; Sun, Peihong; Zhou, Ying; Liu, Yuwang; Zhang, Huilin; Gu, Jingkai; Cui, Yimin (2009). "Pharmacokinetics and safety of aildenafil tablets in healthy Chinese male subjects after multiple dose administration". Zhongguo Linchuang Yaolixue Zazhi. 25 (2): 120–123.
- Liu, Baoshun. Preparation of pyrazolopyrimidine derivatives for treatment of impotence. WO 2003016313
- Gryniewicz, CM; Reepmeyer, JC; Kauffman, JF; Buhse, LF (2009). "Detection of undeclared erectile dysfunction drugs and analogues in dietary supplements by ion mobility spectrometry". Journal of Pharmaceutical and Biomedical Analysis. 49 (3): 601–6. doi:10.1016/j.jpba.2008.12.002. PMID 19150190.
- Choi, Dong Mi; Park, Sangaeh; Yoon, Tae Hyung; Jeong, Hye Kyoung; Pyo, Jae Sung; Park, Janghyun; Kim, Deukjoon; Kwon, Sung Won (2008). "Determination of analogs of sildenafil and vardenafil in foods by column liquid chromatography with a photodiode array detector, mass spectrometry, and nuclear magnetic resonance spectrometry". Journal of AOAC International. 91 (3): 580–588. PMID 18567304.
- Reepmeyer, John C.; Woodruff, Jeffrey T. (2007). "Use of liquid chromatography-mass spectrometry and a chemical cleavage reaction for the structure elucidation of a new sildenafil analogue detected as an adulterant in an herbal dietary supplement". Journal of Pharmaceutical and Biomedical Analysis. 44 (4): 887–893. doi:10.1016/j.jpba.2007.04.011. PMID 17532168.
- Reepmeyer, John C.; Woodruff, Jeffrey T.; 'Avignon, D. Andre. (2007). "Structure elucidation of a novel analogue of sildenafil detected as an adulterant in an herbal dietary supplement". Journal of Pharmaceutical and Biomedical Analysis. 43 (5): 1615–1621. doi:10.1016/j.jpba.2006.11.037. PMID 17207601.
- Enforcement Report for June 30, 2010, United States Food and Drug Administration
- Hidden Risks of Erectile Dysfunction "Treatments" Sold Online, United States Food and Drug Administration, February 21, 2009
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.