Flibanserin
Flibanserin, sold under the trade name Addyi, is a medication approved for the treatment of pre-menopausal women with hypoactive sexual desire disorder (HSDD).[2][3] The medication increases the number of satisfying sexual events per month by about one half over placebo from a starting point of about two to three.[4][5] The certainty of the estimate is low.[4] The side effects of dizziness, sleepiness, and nausea occur about three to four times more often.[4]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Addyi |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 33%[1] |
| Protein binding | ~98% |
| Metabolism | Extensive by liver (mainly by CYP3A4 and CYP2C19) |
| Elimination half-life | ~11 hours |
| Excretion | Biliary (51%), kidney (44%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.170.970 |
| Chemical and physical data | |
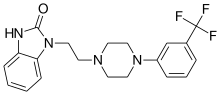
| Formula | C20H21F3N4O |
| Molar mass | 390.40 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Development by Boehringer Ingelheim was halted in October 2010 following a negative evaluation by the U.S. Food and Drug Administration.[6] The rights to the drug were then transferred to Sprout Pharmaceuticals, which achieved approval of the drug by the US FDA in August 2015.[7]
HSDD was recognized as a distinct sexual function disorder for more than 30 years, but was removed from the Diagnostic and Statistical Manual of Mental Disorders in 2013, and replaced with a new diagnosis called female sexual interest/arousal disorder (FSIAD).[8][9]
Medical uses
Flibanserin is used for hypoactive sexual desire disorder among women. Those receiving flibanserin report a 0.5 increase compared to placebo in the number of times they had "satisfying sexual events".[4] In those on flibanserin it rose from 2.8 to 4.5 times a month while women receiving placebo reported also an increase of "satisfying sexual events" from 2.7 to 3.7 times a month.[10] The onset of the flibanserin effect was seen from the first timepoint measured after 4 weeks of treatment and maintained throughout the treatment period.[11][12]
The effectiveness of flibanserin was evaluated in three phase 3 clinical trials. Each of the three trials had two co-primary endpoints, one for satisfying sexual events (SSEs) and the other for sexual desire. Each of the 3 trials also had a secondary endpoint that measured distress related to sexual desire. All three trials showed that flibanserin produced an increase in the number of SSEs and reduced distress related to sexual desire. The first two trials used an electronic diary to measure sexual desire, and did not find an increase. These two trials also measured sexual desire using the Female Sexual Function index (FSFI) as a secondary endpoint, and an increase was observed using this latter measure. The FSFI was used as the co-primary endpoint for sexual desire in the third trial, and again showed a statistically significant increase.[12]
Women's overall feeling of improvement was small to none.[4] The overall quality of the evidence was low.[4]
Side effects
Adverse events are more common among women taking flibanserin. The majority of adverse events were mild to moderate. The most commonly reported adverse events included dizziness, nausea, feeling tired, sleepiness, and trouble sleeping.[13]
Drinking alcohol while on flibanserin may result in severely low blood pressure (low blood pressure that produced symptoms after 2 glasses of wine occurred in 17%).[14]
Mechanism of action
Activity profile
Flibanserin acts as a full agonist in the frontal cortex and the raphe dorsalis, but only as a partial agonist in the CA3 region of the hippocampus[15] of the 5-HT1A receptor (serotonin receptor) (Ki = 1 nM in CHO cells, but only 15–50 nM in cortex, hippocampus and dorsal raphe)[2] and, with lower affinity, as an antagonist of the 5-HT2A receptor (Ki = 49 nM) and antagonist or very weak partial agonist of the D4 receptor (Ki = 4–24 nM).[16][17][18][19] Despite the much greater affinity of flibanserin for the 5-HT1A receptor, and for reasons that are unknown (although it might be caused by the competition with endogenous serotonin), flibanserin occupies the 5-HT1A and 5-HT2A receptors in vivo with similar percentages.[2][20] Flibanserin also has low affinity for the 5-HT2B receptor (Ki = 89.3 nM) and the 5-HT2C receptor (Ki = 88.3 nM), both of which it behaves as an antagonist of.[19] Flibanserin preferentially activates 5-HT1A receptors in the prefrontal cortex, demonstrating regional selectivity, and has been found to increase dopamine and norepinephrine levels and decrease serotonin levels in the rat prefrontal cortex, actions that were determined to be mediated by activation of the 5-HT1A receptor.[16] As such, flibanserin has been described as a norepinephrine–dopamine disinhibitor (NDDI).[19][21]
The proposed mechanism of action refers to the Kinsey dual control model of sexual response.[22] Various neurotransmitters, sex steroids, and other hormones have important excitatory or inhibitory effects on the sexual response. Among neurotransmitters, excitatory activity is driven by dopamine and norepinephrine, while inhibitory activity is driven by serotonin. The balance between these systems is of significance for a normal sexual response. By modulating serotonin and dopamine activity in certain parts of the brain, flibanserin may improve the balance between these neurotransmitter systems in the regulation of sexual response.[23][24]
Society and culture
Flibanserin was originally developed as an antidepressant,[25][26] before being repurposed for the treatment of HSDD.
Names
Former proposed but abandoned trade names of flibanserin include Ectris and Girosa, and its former developmental code name was BIMT-17. The current brand name is Addyi.
Approval process and advocacy
On June 18, 2010, a federal advisory panel to the U.S. Food and Drug Administration (FDA) unanimously voted against recommending approval of flibanserin, citing an inadequate risk-benefit ratio. The Committee acknowledged the validity of hypoactive sexual desire as a diagnosis, but expressed concern with the drug's side effects and insufficient evidence for efficacy, especially the drug's failure to show a statistically significant effect on the co-primary endpoint of sexual desire.[27] Earlier in the week, a FDA staff report also recommended non-approval of the drug. Ahead of the votes, Boehringer Ingelheim had mounted a publicity campaign to promote the controversial disorder of "hypoactive sexual desire".[28] In 2010 the FDA issued a Complete Response Letter, stating that New Drug Application could not be approved in its current form. The letter cited several concerns, including the failure to demonstrate a statistical effect on the co-primary endpoint of sexual desire and overly restrictive entry criteria for the two Phase 3 trials. The Agency recommended performing a new Phase 3 trial with less restrictive entry criteria.[29] On October 8, 2010, Boehringer announced that it would discontinue its development of flibanserin in light of the FDA's decision.[30]
Sprout responded to the FDA's cited deficiencies and refiled the NDA in 2013. The submission included data from a new Phase 3 trial and several Phase 1 drug-drug interaction studies.[29][31] The FDA again refused the application, citing an uncertain risk/benefit ratio. In December 2013, a Formal Dispute Resolution was filed,[32] which contained the requirements of the FDA for further studies. These include two studies in healthy subjects to determine if flibanserin impairs their ability to drive, and to determine if it interferes with other biochemical pathways. The Agency agreed to call a new Advisory Committee meeting to consider whether the risk-benefit ratio of flibanserin was favorable after this additional data was obtained.[32][33][34] Sprout expected to resubmit the New Drug Application (NDA) in the 3rd quarter of 2014.[32][33]
On June 4, 2015, the US FDA Advisory Committee, which includes the Bone, Reproductive, and Urologic Drugs Advisory Committee (BRUDAC) and the Drug Safety and Risk Management Advisory Committee (DSRM), recommended approval of the drug by 18–6, with the proviso that measures be taken to inform women of the drug's side effects.[35][36] On August 18, 2015 the FDA approved Addyi (Flibanserin) for the treatment of premenopausal women with low sexual desire that causes personal distress or relationship difficulties. The approval specified that flibanserin should not be used to treat low sexual desire caused by co-existing psychiatric or medical problems; low sexual desire caused by problems in the relationship; or low sexual desire due to medication side effects.[12]
As of 21 August 2015, The Pharmaceutical Journal reported that Sprout Pharmaceuticals had not yet made an application to the European Medicines Agency for a marketing authorisation.[37]
Advocacy groups
Even the Score, a coalition of women's group's brought together by a Sprout consultant, actively campaigned for the approval of flibanserin. The campaign emphasized that several approved treatments for male sexual dysfunction exist, while no such treatment for women was available.[38] The group successfully obtained letters of support from the President of the National Organization for Women, the editor of the Journal of Sexual Medicine, and several members of Congress.[39]
Other organizations supporting the approval of flibanserin included the National Council of Women's Organizations, the Black Women’s Health Imperative, the Association of Reproductive Health Professionals, National Consumers League, and the American Sexual Health Association.[40][41][42][43]
The approval was opposed by the National Women's Health Network, the National Center for Health Research and Our Bodies Ourselves.[44] A representative of PharmedOut said "To approve this drug will set the worst kind of precedent — that companies that spend enough money can force the FDA to approve useless or dangerous drugs."[45] An editorial in JAMA noted that, "Although flibanserin is not the first product to be supported by a consumer advocacy group in turn supported by pharmaceutical manufacturers, claims of gender bias regarding the FDA’s regulation have been particularly noteworthy, as have the extent of advocacy efforts ranging from social media campaigns to letters from members of Congress".[46]
The Even the Score campaign was managed by Blue Engine Message & Media, a public relations firm, and received funding from Sprout.[47]
Acquisition by Valeant Pharmaceuticals
On 20 August 2015 Valeant Pharmaceuticals and Sprout Pharmaceuticals announced that Valeant will acquire Sprout, on a debt-free basis, for approximately $1 billion in cash, plus a share of future profits based upon the achievement of certain milestones.[48]
Reception
The initial response since the 2015 introduction of flibanserin to the U.S. market was slow with 227 prescriptions written during the first three weeks.[49] The slow response may be related to a number of factors: physicians require an about 10 minute online training to get certified, the medication has to be taken daily and costs about US $400 per month[50], and questions about the drug's efficacy and need.[49] Prescriptions for the drug continue to be few with less than 4,000 being made as of February 2016.[51]
References
- "Addyi™ (flibanserin) Tablets, for Oral Use. Full Prescribing Information" (PDF). Addyi REMS (Risk Evaluation and Mitigation Strategy). Sprout Pharmaceuticals, Inc. Raleigh, NC 27609 USA. Archived from the original (PDF) on 4 March 2016. Retrieved 21 October 2015.
- Borsini F, Evans K, Jason K, Rohde F, Alexander B, Pollentier S (2002). "Pharmacology of flibanserin". CNS Drug Reviews. 8 (2): 117–42. doi:10.1111/j.1527-3458.2002.tb00219.x. PMC 6741686. PMID 12177684.
- Jolly E; Clayton A; Thorp J; Lewis-D’Agostino D; Wunderlich G; Lesko L (April 2008). "Design of Phase III pivotal trials of flibanserin in female Hypoactive Sexual Desire Disorder (HSDD)". Sexologies. 17 (Suppl 1): S133–4. doi:10.1016/S1158-1360(08)72886-X.
- Jaspers, Loes; Feys, Frederik; Bramer, Wichor M.; Franco, Oscar H.; Leusink, Peter; Laan, Ellen T. M. (29 February 2016). "Efficacy and Safety of Flibanserin for the Treatment of Hypoactive Sexual Desire Disorder in Women". JAMA Internal Medicine. 176 (4): 453–62. doi:10.1001/jamainternmed.2015.8565. PMID 26927498.
- "Joint Meeting of the Bone, Reproductive and Urologic Drugs Advisory Committee (BRUDAC) and the Drug Safety and Risk Management (DSaRM) Advisory Committee" (PDF). June 4, 2015. Retrieved 5 June 2015.
- Spiegel online: Pharmakonzern stoppt Lustpille für die Frau, 8 October 2010 (in German)
- Mullard, Asher (1 October 2015). "FDA approves female sexual dysfunction drug". Nature Reviews Drug Discovery. 14 (10): 669. doi:10.1038/nrd4757. PMID 26424353.
- American Psychiatric Association. Sexual and gender identity disorders. In: American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000:493–538.
- Nagoski, Emily (27 February 2015). "Nothing Is Wrong With Your Sex Drive". The New York Times. Retrieved 31 July 2017.
- Jolly E, Thorp J, Clayton AH, et al. Patients’ Perspective of Efficacy of Flibanserin in Premenopausal Women with HSDD. Oral presentation at the 58th Annual Clinical Meeting of The American College of Obstetricians and Gynecologists, May 2010.
- Simon JA, Thorp J, Katz M et al. Onset of Efficacy of Flibanserin in Premenopausal Women with Hypoactive Sexual Desire Disorder. Abstract presented at the 58th Annual Clinical Meeting of The American College of Obstetricians and Gynecologists, May 2010.
- "Highlights of Prescribing Information for Addyi" (PDF).
- "Proposed Indication: Flibanserin is indicated for the treatment of hypoactive sexual desire disorder in premenopausal women" (PDF). May 20, 2010. Retrieved June 16, 2010.
- "FDA Risk Evaluation and Mitigation Strategy of Addyi" (PDF). p. 42.
- Rueter LE, de Montigny C, Blier P (1998). "In vivo electrophysiological assessment of the agonistic properties of flibanserin at pre- and postsynaptic 5-HT1A receptors in the rat brain". Synapse. 29 (4): 392–405. doi:10.1002/(SICI)1098-2396(199808)29:4<392::AID-SYN11>3.0.CO;2-T. PMID 9661257.
- Invernizzi, Roberto William; Sacchetti, Giuseppina; Parini, Stefania; Acconcia, Sabrina; Samanin, Rosario (2003). "Flibanserin, a potential antidepressant drug, lowers 5-HT and raises dopamine and noradrenaline in the rat prefrontal cortex dialysate: role of 5-HT1Areceptors". British Journal of Pharmacology. 139 (7): 1281–1288. doi:10.1038/sj.bjp.0705341. ISSN 0007-1188. PMC 1573953. PMID 12890707.
- Borsini F, Giraldo E, Monferini E, Antonini G, Parenti M, Bietti G, Donetti A (1995). "BIMT 17, a 5-HT2A receptor antagonist and 5-HT1A receptor full agonist in rat cerebral cortex". Naunyn Schmiedebergs Arch. Pharmacol. 352 (3): 276–82. doi:10.1007/bf00168557. PMID 8584042.
- Stahl, Stephen M. (2015). "Mechanism of action of flibanserin, a multifunctional serotonin agonist and antagonist (MSAA), in hypoactive sexual desire disorder". CNS Spectrums. 20 (1): 1–6. doi:10.1017/S1092852914000832. ISSN 1092-8529. PMID 25659981.
- Stahl, Stephen M.; Sommer, Bernd; Allers, Kelly A. (2011). "Multifunctional Pharmacology of Flibanserin: Possible Mechanism of Therapeutic Action in Hypoactive Sexual Desire Disorder". The Journal of Sexual Medicine. 8 (1): 15–27. doi:10.1111/j.1743-6109.2010.02032.x. ISSN 1743-6095. PMID 20840530.
- Scandroglio A, Monferini E, Borsini F (2001). "Ex vivo binding of flibanserin to serotonin 5-HT1A and 5-HT2A receptors". Pharmacol. Res. 43 (2): 179–83. doi:10.1006/phrs.2000.0762. PMID 11243720.
- Stephen M. Stahl; S. M. Stahl (17 March 2008). Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge University Press. p. 658. ISBN 978-0-521-67376-1. Retrieved 23 April 2012.
- Janssen, E, Bancroft J. The dual control model: The role of sexual inhibition & excitation in sexual arousal and behavior In Janssen, E. (Ed). (2006). The Psychophysiology of Sex. Bloomington, IN:Indiana University press.
- Pfaus JG (June 2009). "Pathways of sexual desire". J Sex Med. 6 (6): 1506–33. doi:10.1111/j.1743-6109.2009.01309.x. PMID 19453889.
- Allers K, Dremencov E, Ceci A, et al. (May 2010). "Acute and repeated flibanserin administration in female rats modulates monoamines differentially across brain areas: a microdialysis study". J Sex Med. 7 (5): 1757–67. doi:10.1111/j.1743-6109.2010.01763.x. PMID 20163532.
- D'Aquila P, Monleon S, Borsini F, Brain P, Willner P (December 1997). "Anti-anhedonic actions of the novel serotonergic agent flibanserin, a potential rapidly-acting antidepressant". European Journal of Pharmacology. 340 (2–3): 121–32. doi:10.1016/S0014-2999(97)01412-X. PMID 9537806.
- Invernizzi RW, Sacchetti G, Parini S, Acconcia S, Samanin R (August 2003). "Flibanserin, a potential antidepressant drug, lowers 5-HT and raises dopamine and noradrenaline in the rat prefrontal cortex dialysate: role of 5-HT1A receptors". Br J Pharmacol. 139 (7): 1281–8. doi:10.1038/sj.bjp.0705341. PMC 1573953. PMID 12890707.
- "June 18, 2010 meeting of the FDA Advisory Committee for Reproductive Health Drugs" (PDF), Minutes, retrieved 2015-11-18
- "Drug for sexual desire disorder opposed by panel". New York Times. 18 June 2010.
- "Joint Meeting of the Bone, Reproductive and Urologic Drugs Advisory Committee (BRUDAC) and the Drug Safety and Risk Management (DSaRM) Advisory Committee" (PDF).
- Burger, Ludwig (8 October 2010). "Boehringer pulls the plug on "pink Viagra"". Reuters.
- "Sprout Pharmaceuticals resubmits flibanserin NDA for treating HSDD in pre-menopausal women". 27 June 2013.
- "ADDYI® (flibanserin) - Home". sproutpharma.com. Retrieved 31 July 2017.
- FDA seeks more tests on a female Viagra, by Matthew Perrone, The Detroit Free Press, page 2A Wednesday, Feb. 12, 2014
- CNN, By Elizabeth Landau. "FDA: Female sex drive drug needs more research - CNN.com". CNN. Retrieved 31 July 2017.
- Stein, Rob (June 4, 2015). "Advisers To FDA Recommend Agency Approve Drug To Boost Female Libido". NPR. Retrieved June 4, 2015.
- "Critics: Women's Sex Pill Approval Vote Driven By PR, Not Science". Forbes. June 7, 2015.
- Torjesen I (21 August 2015). "First drug to improve sexual desire in women approved in the United States". The Pharmaceutical Journal. 295 (7878). doi:10.1211/PJ.2015.20069201.
- Pollack, Andrew (2015-06-04). "'Viagra for Women' Is Backed by an F.D.A. Panel". The New York Times.
- "Why Flibanserin Is Not the 'Female Viagra' - The Atlantic".
- "F.D.A. Approves Addyi, a Libido Pill for Women - The New York Times".
- "Association of Reproductive Health Professionals". Retrieved 2015-11-17.
- "National Consumers League". Retrieved 2015-11-17.
- "American Sexual Health Association". Retrieved 2015-11-17.
- "Raleigh's Sprout Pharmaceuticals awaits FDA ruling on female libido drug | News & Observer".
- Perry, Susan (8 June 2015). "'Faux-advocacy,' not science, prompted FDA panel's OK of 'low libido' drug for women, critics charge". minnpost.com. Retrieved 18 August 2015.
- Gellad WF, Flynn KE, Alexander GC (2015). "Evaluation of Flibanserin: Science and Advocacy at the FDA". JAMA. 314 (9): 869–70. doi:10.1001/jama.2015.8405. PMID 26148201.
- Karlin, Sarah (13 August 2015). "Women's sex drug gets political hard sell". politico.com. Retrieved 18 August 2015.
- "Archived copy". Archived from the original on 2015-08-22. Retrieved 2015-10-25.CS1 maint: archived copy as title (link)
- Anna Edney; Laura Colbey (November 17, 2015). "The Female Libido Pill Is No Viagra". Bloomberg Business. Retrieved November 18, 2015.
- "Addyi Flibanserin". GoodRx.
- Thomas, Katie. "The Female Viagra, Undone by a Drug Maker's Dysfunction". The New York Times. Retrieved 9 April 2016.
External links
- Yves Aubert, Thesis, Leiden University. (Dec 11, 2012) Sex, aggression and pair-bond: a study on the serotonergic regulation of female sexual function in the marmoset monkey
- Viagra for women? (at Businessweek)
- Marazziti D, Palego L, Giromella A, et al. (June 2002). "Region-dependent effects of flibanserin and buspirone on adenylyl cyclase activity in the human brain". Int. J. Neuropsychopharmacol. 5 (2): 131–40. doi:10.1017/S1461145702002869. PMID 12135537.
- Podhorna J, Brown RE (June 2000). "Flibanserin has anxiolytic effects without locomotor side effects in the infant rat ultrasonic vocalization model of anxiety". Br J Pharmacol. 130 (4): 739–746. doi:10.1038/sj.bjp.0703364. PMC 1572126. PMID 10864879. Archived from the original on 2013-01-06.
- Brambilla A, Baschirotto A, Grippa N, Borsini F (December 1999). "Effect of flibanserin (BIMT 17), fluoxetine, 8-OH-DPAT and buspirone on serotonin synthesis in rat brain". Eur Neuropsychopharmacol. 10 (1): 63–7. doi:10.1016/S0924-977X(99)00056-5. PMID 10647099.
- "FDA orders important safety labeling changes for Addyi". The FDA. Retrieved 11 April 2019.
- "The Company Behind 'Female Viagra' Just Raised $20 Million in Funding". Fortune. Retrieved 4 September 2019.