Terguride
Terguride (INN), also known as trans-dihydrolisuride, is a serotonin receptor antagonist and dopamine receptor agonist of the ergoline family. It is approved for and used as a prolactin inhibitor in the treatment of hyperprolactinemia. Terguride is an oral, potent antagonist of 5-HT2B and 5-HT2A (serotonin) receptors. Serotonin stimulates the proliferation of pulmonary artery smooth muscle cells, and induces fibrosis in the wall of pulmonary arteries. Together, this causes vascular remodeling and narrowing of the pulmonary arteries. These changes result in increased vascular resistance and PAH. Due to the potential anti-proliferative and anti-fibrotic activity of terguride, this potential medicine could offer the hope of achieving reversal of pulmonary artery vascular remodeling and attenuation of disease progression.[1]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.732 |
| Chemical and physical data | |
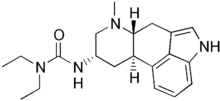
| Formula | C20H28N4O |
| Molar mass | 340.46 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
In May 2008, terguride was granted orphan drug status for the treatment of pulmonary arterial hypertension.[2] In May 2010 Pfizer purchased worldwide rights for the drug.[3]
References
- Janssen W, Schymura Y, Novoyatleva T, Kojonazarov B, Boehm M, Wietelmann A, et al. "5-HT2B receptor antagonists inhibit fibrosis and protect from RV heart failure". BioMed Research International. 2015: 438403. doi:10.1155/2015/438403. PMID 25667920.
- Presseportal (Swiss press portal, in German)
- TheDay.com 5/10/2010
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Lysergic acid derivatives |
|
|---|---|
| Psychedelic lysergamides | |
| clavines |
|
| Other ergolines | |
| Natural sources |
Morning glory: Argyreia nervosa (Hawaiian Baby Woodrose), Ipomoea spp.(Morning Glory, Tlitliltzin, Badoh Negro), Rivea corymbosa (Coaxihuitl, Ololiúqui) |