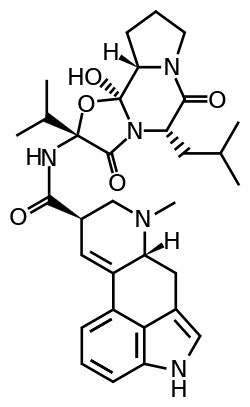
Ergocryptine
Ergocryptine is an ergopeptine and one of the ergot alkaloids. It is isolated from ergot or fermentation broth and it serves as starting material for the production of bromocriptine.[1]
 | |
| Identifiers | |
|---|---|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| ChEMBL | |
| ECHA InfoCard | 100.007.384 |
| Chemical and physical data | |
| Formula | C32H41N5O5 |
| Molar mass | 575.698 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Chemistry
Ergocryptine is a mixture of two very similar compounds, alpha- and beta-ergocryptine.[2] The beta differs from the alpha form only in the position of a single methyl group, which is a consequence of the biosynthesis in which the proteinogenic amino acid leucine is replaced by isoleucine.
Biosynthesis
The biosynthetic pathways to ergocryptine starts with the prenylation of L-tryptophan in an SN1 fashion with Dimethylallylpyrophosphate (DMAPP). The DMAPP is derived from Mevalonic Acid. This reaction is catalyzed by a prenyltransferase enzyme (Prenyltransferase 4-dimethylallyltryptophan synthase) named FgaPT2 in A. fumigatus.[3][4] An X-ray structure of the prenyltransferase FgaPT2 and tryptophan has been reported, and used to propose a three step mechanism (formation of allylic carbocation, nucleophilic attack of tryptophan on the carbocation, then deprotonation to restore aromaticity and generate the product, 4-dimethylallyltryptophan (DMAT)).[5] DMAT is then N-methylated at the amino of the tryptophan backbone with the EasF enzyme, named FgaMT in A. fumigatus. S-adenosylmethionine (SAM) being the methyl source.[6]
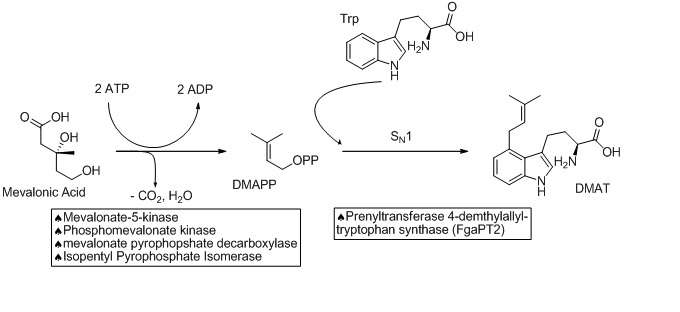
The next step in the biosynthesis of ergocryptine is the transformation of 4-dimethylallyl abrine to Chanoclavine-I. It has been shown that the enzymes EasE and EasC (FgaOx1 and FgaCat in A. fumigatus, respectively) are both required to generate Chanoclavine-I from 4-DMA abrine.[7] Mutation experiments altering these enzymes independently stopped the pathway at abrine. This indicates that cooperation between EasE and EasC is necessary.
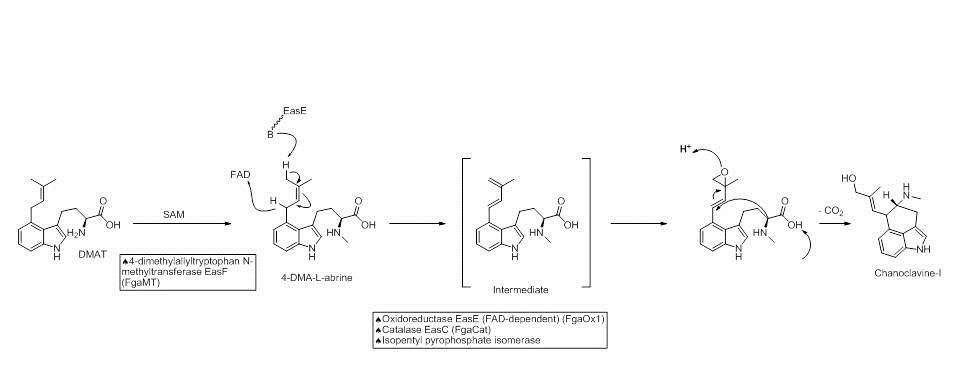
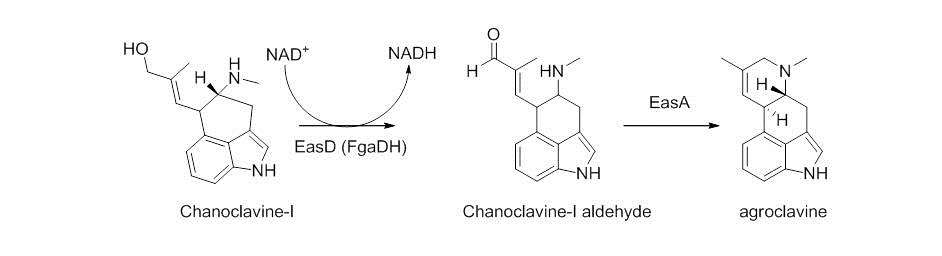
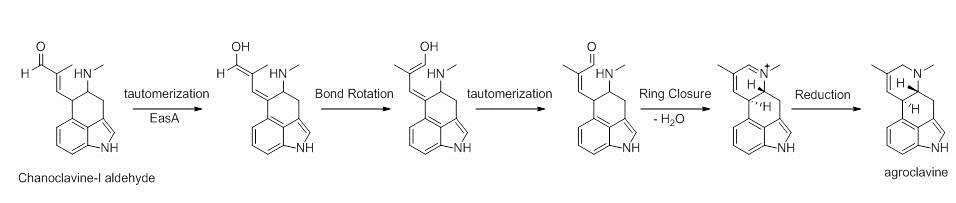
Chanocalvine-I is then oxidized to chanoclavine-I aldehyde with NAD+ dependent enzyme EasD (FgaDH in A. fumigatus). Chanoclavine-I aldehyde is a branch point, leading to different ergot alkaloids, depending on the specific fungus. In C. purpurea, chanoclavine-I aldehyde is converted to argoclavine with EasA, referred to as the old yellow enzyme or FgaOx3. This process occurs via keto-enol tautomerization to facilitate rotation about a carbon-carbon bond, followed by tautomerization back to the aldehyde, and condensation with the proximal secondary amine.[8][9] The iminium species created by cyclization is then reduced to the tertiary amine, yielding agroclavine.
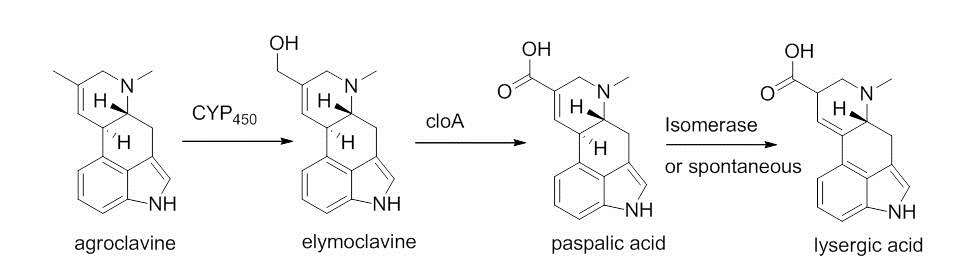
A cyctochrome P-450 monooxygenase enzyme catalyzes a two electron oxidation of agroclavne to the corresponding primary alcohol, elymoclavine.[10] Elymoclavine is then oxidized by four electrons by a P450 monooxygenase to give paspalic acid. Paspalic acid then undergoes isomerization of the carbon-carbon double bond that is in conjugation with the acid, to give D-lysergic acid.
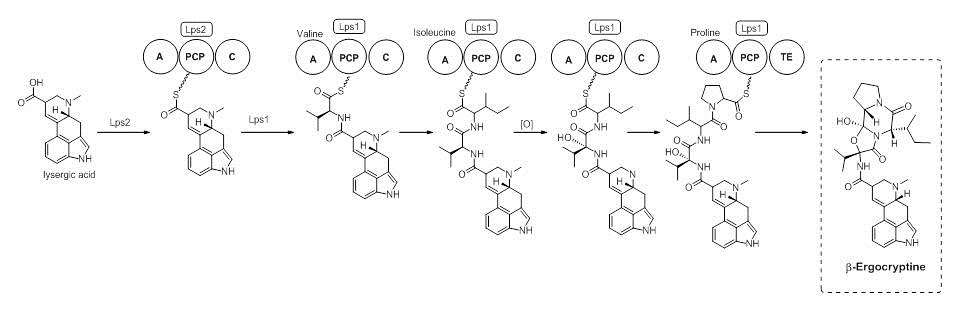
Lysergic Acid is a branch point in the biosynthesis of ergoamides and ergopeptines. On the path to ergocryptine, an ergopeptine, the tripeptide is installed by a Non-Ribosomal Peptide Synthase (NRPS). It has been shown that there are two enzymes, D-lysergyl peptide synthases (LPS) 1 and 2, which are responsible for the tripeptide connection to lysergic acid.[11] The timing of the oxidation of valine to an alcohol is not exactly known. However, it is speculated that the oxidation occurs while bound to the NRPS LPS2.[12] Ergocryptine is found in two forms, differing in the amino acid used by the NRPS. The alpha form contains the amino acid leucine, while the beta-form uses the amino acid isoleucine.[13]
See also
References
- Kren V., Cvak L. "Ergot: the genus Claviceps". Amsterdam: Harwood Academic Publishers; 1999. p. 399–401
- Yates, S. G.; Plattner, R. D.; Garner, G. B. (1985). "Detection of ergopeptine alkaloids in endophyte-infected, toxic Ky-31 tall fescue by mass spectrometry/mass spectrometry" (PDF). Journal of Agricultural and Food Chemistry. 33 (4): 719. doi:10.1021/jf00064a038.
- Floss, H. G.; Heinstein, P. Purification and Properties Biosynthesis of Dimethylallylpyrophosphate : Tryptophan the First Enzyme of Ergot Alkaloid in Claviceps . Dimethylallyl. Arch. Biochem. Biophys. 1976, 94, 84–94.
- Gerhards, N.; Neubauer, L.; Tudzynski, P.; Li, S.-M. Biosynthetic Pathways of Ergot Alkaloids. Toxins (Basel). 2014, 6, 3281–3295.
- Gerhards, N.; Neubauer, L.; Tudzynski, P.; Li, S.-M. Biosynthetic Pathways of Ergot Alkaloids. Toxins (Basel). 2014, 6, 3281–3295.
- Rigbers, O.; Li, S. M. Ergot Alkaloid Biosynthesis in Aspergillus Fumigatus: Overproduction and Biochemical Characterization of a 4-Dimethylallyltryptophan N-Methyltransferase. J. Biol. Chem. 2008, 283, 26859–26868.
- Goetz, K. E.; Coyle, C. M.; Cheng, J. Z.; O'Connor, S. E.; Panaccione, D. G. Ergot Cluster-Encoded Catalase Is Required for Synthesis of Chanoclavine-I in Aspergillus Fumigatus. Curr. Genet. 2011, 57, 201–211.
- Gerhards, N.; Neubauer, L.; Tudzynski, P.; Li, S.-M. Biosynthetic Pathways of Ergot Alkaloids. Toxins (Basel). 2014, 6, 3281–3295.
- Coyle, C. M.; Cheng, J. Z.; O’Connor, S. E.; Panaccione, D. G. An Old Yellow Enzyme Gene Controls the Branch Point between Aspergillus Fumigatus and Claviceps Purpurea Ergot Alkaloid Pathways. Appl. Environ. Microbiol. 2010, 76, 3898–3903.
- Haarmann, T.; Machado, C.; Lübbe, Y.; Correia, T.; Schardl, C. L.; Panaccione, D. G.; Tudzynski, P. The Ergot Alkaloid Gene Cluster in Claviceps Purpurea: Extension of the Cluster Sequence and Intra Species Evolution. Phytochemistry 2005, 66, 1312–1320.
- Walzel, B.; Riederer, B.; Keller, U. Mechanism of Alkaloid Cyclopeptide Synthesis in the Ergot Fungus Claviceps Purpurea. Chem. Biol. 1997, 4, 223–230.
- Keller, U. Genetics and Biochemistry of Antibiotic Production; Butterworth-Heinemann: Boston, 1995.
- Gerhards, N.; Neubauer, L.; Tudzynski, P.; Li, S.-M. Biosynthetic Pathways of Ergot Alkaloids. Toxins (Basel). 2014, 6, 3281–3295.