TFMFly
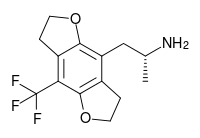
TFMFly is a compound related to psychedelic phenethylamines such as 2C-B-FLY and 2C-TFM. It was first reported in 2005 by a team at Purdue University led by David Nichols.[1] It acts as a potent agonist at the 5HT2A serotonin receptor subtype, and is a chiral compound with the more active (R) enantiomer having a Ki of 0.12nM at the human 5HT2A receptor.[2] While the fully aromatic benzodifurans such as bromodragonfly generally have higher binding affinity than saturated compounds like 2C-B-FLY,[3] the saturated compounds have higher efficacy as agonists.[4]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C14H16F3NO2 |
| Molar mass | 287.277 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Legal Status
TFMFly is illegal in Latvia.[5]
References
- Michael Robert Braden PhD. Towards a biophysical understanding of hallucinogen action. Purdue University 2007.
- Parrish JC, Braden MR, Gundy E, Nichols DE (December 2005). "Differential phospholipase C activation by phenylalkylamine serotonin 5-HT 2A receptor agonists". Journal of Neurochemistry. 95 (6): 1575–84. doi:10.1111/j.1471-4159.2005.03477.x. PMID 16277614.
- Chambers JJ, Kurrasch-Orbaugh DM, Parker MA, Nichols DE (March 2001). "Enantiospecific synthesis and pharmacological evaluation of a series of super-potent, conformationally restricted 5-HT(2A/2C) receptor agonists". Journal of Medicinal Chemistry. 44 (6): 1003–10. CiteSeerX 10.1.1.691.362. doi:10.1021/jm000491y. PMID 11300881.
- Ralf Heim PhD. Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. Entwicklung eines neuen Struktur-Wirkungskonzepts. (German)
- Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.