Lumateperone
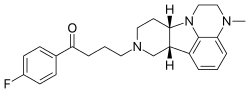
Lumateperone (INN; developmental codes ITI-007 and ITI-722) is an investigational butyrophenone antipsychotic which is currently under development by Intra-Cellular Therapies, licensed from Bristol-Myers Squibb, for the treatment of schizophrenia,[1] as well as for bipolar depression and other neurological indications.[2]
 | |
| Clinical data | |
|---|---|
| Other names | ITI-007; ITI-722 |
| Routes of administration | By mouth |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C24H28FN3O |
| Molar mass | 393.506 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Clinical Studies
Schizophrenia
Bipolar depression
Two phase 3 monotherapy Lumapteperone studies were conducted and completed for the treatment of bipolar depression, those being trial Study 401 and Study 404[3]. A third trial, Study 402, aims to test Lumateperone in addition to lithium or valporate[4][5], the data pertaining this trial is due out in 2020.[6][7]
Study 401 was conducted solely in the United States, while Study 404 was a global study and included patients from the US.[8][9] Of the entire Study 404 population (381 patients), two-thirds were from Russia and Colombia. At the completion of the two monotherpay phase 3 trials, only Study 404 met its primary endpoint, and one of its secondary endpoints.[10][11] In Study 404, patients received 42mg of Lumateperone, once daily, or placebo for six weeks. Study 404 patients, saw a betterment of depressive symptoms compared to placebo, as documented by a change in MADRS total score of 4.6.[12]
Lumateperone and Intra-Cellular Stock
The failure of Study 401, caused Intra-Cellular's stock price to fall.[13][14] Their stock fell again on July 23rd when the FDA cancelled a Psychopharmacologic Drugs Advisory Committee meeting.[15][16]
References
- Sylvain Celanire; Sonia Poli (13 October 2014). Small Molecule Therapeutics for Schizophrenia. Springer. pp. 31–. ISBN 978-3-319-11502-3.
- "Another blow for Intra-Cellular". Evaluate.com. 2019-07-24. Retrieved 2019-11-06.
- Inc, Intra-Cellular Therapies (2019-07-08). "Intra-Cellular Therapies Announces Positive Top-line Results from a Phase 3 Trial of Lumateperone in Patients with Bipolar Depression". GlobeNewswire News Room. Retrieved 2019-11-06.
- Inc, Intra-Cellular Therapies (2019-07-08). "Intra-Cellular Therapies Announces Positive Top-line Results from a Phase 3 Trial of Lumateperone in Patients with Bipolar Depression". GlobeNewswire News Room. Retrieved 2019-11-06.
- "Why Intra-Cellular Therapies Is Tanking Today". finance.yahoo.com. Retrieved 2019-11-06.
- "One out of two is not enough for Intra-Cellular". Evaluate.com. 2019-07-08. Retrieved 2019-11-06.
- "Why Intra-Cellular Therapies Is Tanking Today". finance.yahoo.com. Retrieved 2019-11-06.
- "Intra-Cellular Therapies Announces Top Line Results for Two Bipolar Studies". Trial Site News. 2019-07-13. Retrieved 2019-11-06.
- Inc, Intra-Cellular Therapies (2019-07-08). "Intra-Cellular Therapies Announces Positive Top-line Results from a Phase 3 Trial of Lumateperone in Patients with Bipolar Depression". GlobeNewswire News Room. Retrieved 2019-11-06.
- "One out of two is not enough for Intra-Cellular". Evaluate.com. 2019-07-08. Retrieved 2019-11-06.
- DeArment, Alaric (2019-07-08). "Intra-Cellular Therapies hits one, misses another in Phase III bipolar disorder program". MedCity News. Retrieved 2019-11-06.
- July 8; 2019. "Phase 3 data supports lumateperone for bipolar depression". www.healio.com. Retrieved 2019-11-06.
- House, SA Editor Douglas W. (2019-07-08). "Intra-Cellular down 9% premarket on uneven results from lumateperone studies". Seeking Alpha. Retrieved 2019-11-06.
- "Why Intra-Cellular Therapies Is Tanking Today". finance.yahoo.com. Retrieved 2019-11-06.
- "Lumateperone schizophrenia drug seems to hit snag". www.mdedge.com. Retrieved 2019-11-06.
- "Lumateperone for schizophrenia shows safety, tolerability in long-term study". www.mdedge.com. Retrieved 2019-11-06.