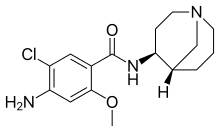
Renzapride
Renzapride is a gastroprokinetic agent and antiemetic which acts as a full 5-HT4 full agonist and 5-HT3 antagonist.[1][2] It also functions as a 5-HT2B antagonist and has some affinity for the 5-HT2A and 5-HT2C receptors.[1]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C16H22ClN3O2 |
| Molar mass | 323.818 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Renzapride was being developed by Alizyme plc of the United Kingdom. In May 2016, EndoLogic LLC, a US based pharmaceutical and medical device company acquired the US and world wide patent rights to Renzapride. EndoLogic planned to develop Renzapride for the treatment of gastroparesis.
Gastroparesis is a common condition affecting more than 20 million people in the US including 5 million diabetics. Currently, only one drug, metoclopramide, dopamine D2 receptor antagonist, is FDA approved for the treatment of gastroparesis in the US.
Patients treated with metoclopramide are at risk for serious side effects, some of which are permanent, such as tardive dyskinesia, hence limiting the use of metoclopramide to no more than 12 weeks.
Endologic confirmed the cardiac safety of renzapride through a “Thorough QTc” study [3] and sold the rights to Atlantic Healthcare in 2019.[4]
Clinical trials
Renzapride was being investigated for the treatment of constipation-predominant irritable bowel syndrome (IBS-C). It is also potentially effective for irritable bowel syndrome with alternating stool pattern (IBS-A). It is being developed by Alizyme plc of the United Kingdom.
The renzapride Phase 3 trial in 2008 [5]for the treatment of constipation-dominant irritable bowel syndrome (IBS-C) demonstrated a small but statistically significant benefit in the Phase 3 study in IBS-C, however, Alizyme decided not to pursue development of the drug for this indication.
References
- Meyers NL, Hickling RI (2008). "Pharmacology and metabolism of renzapride : a novel therapeutic agent for the potential treatment of irritable bowel syndrome". Drugs in R&D. 9 (1): 37–63. doi:10.2165/00126839-200809010-00004. PMID 18095752.
- Camilleri, M.; McKinzie, S.; Fox, J.; Foxxorenstein, A.; Burton, D.; Thomforde, G.; Baxter, K.; Zinsmeister, A. (2004). "Effect of renzapride on transit in constipation-predominant irritable bowel syndrome". Clinical Gastroenterology and Hepatology. 2 (10): 895–904. doi:10.1016/s1542-3565(04)00391-x.
- "FDA accepts cardiac safety trial for gastroparesis drug".
- "Atlantic Healthcare pounces on big bucks US opportunity". Business Weekly.
- George, A. M.; Meyers, N. L.; Hickling, R. I. (2008). "Clinical trial: Renzapride therapy for constipation-predominant irritable bowel syndrome - multicentre, randomized, placebo-controlled, double-blind study in primary healthcare setting". Alimentary Pharmacology & Therapeutics. 27 (9): 830–837. doi:10.1111/j.1365-2036.2008.03649.x. PMID 18284648.