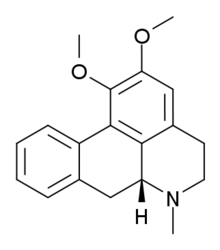
Nuciferine
Nuciferine is an alkaloid found within the plants Nymphaea caerulea and Nelumbo nucifera.
 | |
| Names | |
|---|---|
| IUPAC name
(6aR)-1,2-dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline | |
| Other names
(R)-1,2-Dimethoxyaporphine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C19H21NO2 |
| Molar mass | 295.376 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preliminary psychopharmacological research in 1978 was unable to conclusively determine the compound's classification in regards to dopamine-receptor activity.[1]
On one hand, investigative studies found evidence of behavior traditionally associated with dopamine-receptor stimulation: stereotypy, increase in spontaneous motor activity, inhibition of conditioned avoidance response, and an increase in pain sensitivity resulting in an inhibition of morphine analgesia.[1]
On the other hand, these early investigative studies also found evidence of behavior traditionally associated with dopamine-receptor blockade: decrease of spontaneous motor activity, chills, catalepsy, trance-like states of consciousness.[1]
Nuciferine may also potentiate morphine analgesia. The median lethal dose in mice is 289 mg/kg. It is structurally related to apomorphine.[2][3]
Nuciferine has been reported to have various anti-inflammatory effects. Specific tests found nuciferine to reduce inflammation on lipopolysaccharide (LPS)-stimulated BV2 microglia cells. Researchers proposed that Nuciferine may have activated PPAR-y pathways to inhibit inflammation. More research into these pathways is necessary for a conclusive model for the anti-inflammatory response seen in laboratory studies.[4]
Nuciferine is a potential treatment for liver disease in Type-2 diabetic patients. In diabetic animal models with a high-fat diet, Nuciferine reversed liver damage. The underlying mechanism is unclear. The method of administration was a simple food additive. Culinary methods of preparing lotus flower (a botanical source of naturally-occurring Nuciferine) make this a potentially viable preventative method to prevent liver damage with diet.[5]
Nuciferine's ability to penetrate the blood brain barrier, makes it an interesting candidate for treating diseases of the brain. Various anti-tumor and anti-viral pharmacological properties have been investigated. Nuciferine was shown to inhibit the progression of glioblastoma cancer cells by suppressing the SOX2-AKT/STAT3 signaling pathway in animal models. Targeting SOX-2 pathways in human models with Nuciferine may offer a novel therapeutic approach for cancer treatment.[6]
Nuciferine has shown efficacy as a treatment for premature ejaculation and erectile dysfunction.[7]
The unique properties of this compound correlate with poetry for the botanical sources Nymphaea caerulea and Nelumbo nucifera. These traditionally sacred plants are used in spiritual rituals for healing and enlightenment. In Egyptian mystic art, it's referred to as Sacred Lotus of the Nile. In Buddhist mystic art, it's referred to as the utpala.
A comprehensive scientific model of consciousness has yet to be determined. Testing the effects of Nymphaea caerulea and spirit communication is difficult to model for scientific laboratory studies. Sleep Studies may provide a model to test these unique psychopharmacological features.
See also
- Apomorphine
- Aporphine
- Glaucine
- Bulbocapnine
- Nantenine
- Pukateine
- Stepholidine
- Tetrahydropalmatine
References
- Bhattacharya, S. K.; Bose, R.; Ghosh, P.; Tripathi, V. J.; Ray, A. B.; Dasgupta, B. (1978-09-15). "Psychopharmacological studies on (--)-nuciferine and its Hofmann degradation product atherosperminine". Psychopharmacology. 59 (1): 29–33. doi:10.1007/bf00428026. ISSN 0033-3158. PMID 100809.
- Bhattacharya SK, Bose R, Ghosh P, Tripathi VJ, Ray AB, Dasgupta B (Sep 1978). "Psychopharmacological studies on (—)-nuciferine and its Hofmann degradation product atherosperminine". Psychopharmacology. 59 (1): 29–33. doi:10.1007/BF00428026. PMID 100809.
- Spess, David L. Errors in Alkaloids of Nelumbo and Nymphaea species, 2011, academia.edu
- Zhang, Lina; Gao, Jinghua; Tang, Peng; Chong, Li; Liu, Yue; Liu, Peng; Zhang, Xin; Chen, Li; Hou, Chen (October 2018). "Nuciferine inhibits LPS-induced inflammatory response in BV2 cells by activating PPAR-γ". International Immunopharmacology. 63: 9–13. doi:10.1016/j.intimp.2018.07.015. ISSN 1878-1705. PMID 30056259 – via PubMed.
- Zhang, Chao; Deng, Jianjun; Liu, Dan; Tuo, Xingxia; Xiao, Lei; Lai, Baochang; Yao, Qinyu; Liu, Jia; Yang, Haixia; Wang, Nanping (November 2018). "Nuciferine ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through a PPARα/PPARγ coactivator-1α pathway". British Journal of Pharmacology. 175 (22): 4218–4228. doi:10.1111/bph.14482. ISSN 1476-5381. PMC 6193881. PMID 30129056 – via PubMed.
- Li, Zizhuo; Chen, Yaodong; An, Tingting; Liu, Pengfei; Zhu, Jiyuan; Yang, Haichao; Zhang, Wei; Dong, Tianxiu; Jiang, Jian; Zhang, Yu; Jiang, Maitao (2019-03-29). "Nuciferine inhibits the progression of glioblastoma by suppressing the SOX2-AKT/STAT3-Slug signaling pathway". Journal of experimental & clinical cancer research: CR. 38 (1): 139. doi:10.1186/s13046-019-1134-y. ISSN 1756-9966. PMC 6440136. PMID 30922391 – via PubMed.
- Cai, Tommaso; Cocci, Andrea; Cito, Gianmartino; Giammusso, Bruno; Zucchi, Alessandro; Chiancone, Francesco; Carrino, Maurizio; Mastroeni, Francesco; Comerci, Francesco; Franco, Girgio; Palmieri, Alessandro (2018-03-31). "The role of diallyl thiosulfinate associated with nuciferine and diosgenin in the treatment of premature ejaculation: A pilot study". Archivio Italiano Di Urologia, Andrologia: Organo Ufficiale [di] Societa Italiana Di Ecografia Urologica E Nefrologica. 90 (1): 59–64. doi:10.4081/aiua.2018.1.59. ISSN 1124-3562. PMID 29633800 – via PubMed.