Lophophine
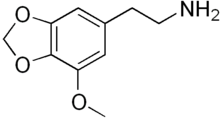
Lophophine (MMDPEA or 3-methoxy-4,5-methylenedioxyphenethylamine) is a putative psychedelic and entactogen drug of the methylenedioxyphenethylamine class. It is the α-demethylated homologue of MMDA, and is also closely related to mescaline.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.645 |
| Chemical and physical data | |
| Formula | C10H13NO3 |
| Molar mass | 195.218 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Alexander Shulgin originally suggested that lophophine may be a natural constituent of peyote (Lophophora williamsii) due to it being the only logical chemical intermediate for the biosynthesis of several tetrahydroisoquinolines known to be present in this cactus species.[1] Subsequently, lophophine was indeed shown to be a minor component of both peyote and San Pedro cactus.[2]
Shulgin reports that lophophine is active in the dosage range of 150–250 mg. He states that at these doses, lophophine has some similarity to mescaline in action, in producing a peaceful elevation of mood, euphoria, and mild enhancement of visual perception, but without the generation of closed-eye mental imagery. Shulgin also notes that (in contrast to mescaline), lophophine causes no nausea.[1]
See also
References
- A. Shulgin and A. Shulgin (1991). Pihkal. Berkeley: Transform Press. pp. 701–702.
- Bruhn JG, El-Seedi HR, Stephanson N, Beck O, Shulgin AT (June 2008). "Ecstasy analogues found in cacti". Journal of Psychoactive Drugs. 40 (2): 219–22. CiteSeerX 10.1.1.689.4014. doi:10.1080/02791072.2008.10400635. PMID 18720674.
External links
- PiHKAL: #95 Lophophine: 3-Methoxy-4,5-methylenedioxyphenethylamine
- Mescaline: The Chemistry and Pharmacology of its Analogues
Empathogens/entactogens | |
|---|---|
| Phenylalkyl- amines (other than cathinones) |
|
| Cyclized phenyl- alkylamines | |
| Cathinones |
|
| Tryptamines | |
| Chemical classes | |
| DRAs |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAs |
| ||||||||||||||
| SRAs |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine reuptake inhibitors • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
Drugs described in PiHKAL | |
|---|---|
|