5-MeO-NBpBrT
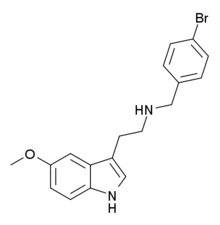
5-MeO-NBpBrT (5-Methoxy-N-(4-bromobenzyl)tryptamine) is a N-substituted member of the methoxytryptamine family of compounds. Like other such compounds it acts as an antagonist for the 5-HT2A receptor, with a claimed 100x selectivity over the closely related 5-HT2C receptor.[1] While N-benzyl substitution of psychedelic phenethylamines often results in potent 5-HT2A agonists, it had been thought that N-benzyl tryptamines show much lower efficacy and are either very weak partial agonists or antagonists at 5-HT2A,[2][3] though more recent research has shown stronger agonist activity for 3-substituted benzyl derivatives.[4] Extending the benzyl group to a substituted phenethyl can also recover agonist activity in certain cases.[5]
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H19BrN2O |
| Molar mass | 359.260 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
References
- Glennon RA, Dukat M, el-Bermawy M, Law H, De los Angeles J, Teitler M, et al. (June 1994). "Influence of amine substituents on 5-HT2A versus 5-HT2C binding of phenylalkyl- and indolylalkylamines". Journal of Medicinal Chemistry. 37 (13): 1929–35. doi:10.1021/jm00039a004. PMID 8027974.
- Silva M (2009). Theoretical study of the interaction of agonists with the 5-HT2A receptor (PhD thesis). Universität Regensburg.
- Silva ME, Heim R, Strasser A, Elz S, Dove S (January 2011). "Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor". Journal of Computer-Aided Molecular Design. 25 (1): 51–66. CiteSeerX 10.1.1.688.2670. doi:10.1007/s10822-010-9400-2. PMID 21088982.
- Nichols DE, Sassano MF, Halberstadt AL, Klein LM, Brandt SD, Elliott SP, Fiedler WJ (July 2015). "N-Benzyl-5-methoxytryptamines as Potent Serotonin 5-HT2 Receptor Family Agonists and Comparison with a Series of Phenethylamine Analogues". ACS Chemical Neuroscience. 6 (7): 1165–75. doi:10.1021/cn500292d. PMID 25547199.
- Jensen N (2004). Tryptamines as Ligands and Modulators of the Serotonin 5-HT2A Receptor and the Isolation of Aeruginascin from the Hallucinogenic Mushroom Inocybe aeruginascens (PDF) (PhD thesis). Georg-August-Universität zu Göttingen.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.