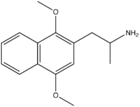
Ganesha (psychedelic)
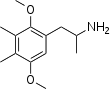
Ganesha (2,5-dimethoxy-3,4-dimethylamphetamine) is a lesser-known psychedelic drug. It is also a substituted amphetamine. It was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage range is listed as 24–32 mg.[1] The drug is usually taken orally, although other routes such as rectally may also be used. Ganesha is synthesized from 2,5-dimethoxy-3,4-dimethylbenzaldehyde. Ganesha is the amphetamine analog of 2C-G. It is a particularly long lasting drug, with the duration listed in PiHKAL as being 18–24 hours, which might make it undesirable to some users. It is named after the Hindu deity, Ganesha. Very little is known about the dangers or toxicity of ganesha. Effects of ganesha include:[1]
- Strong closed-eye visuals
- An increased appreciation of music
- Powerful relaxation and tranquility
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-(2,5-Dimethoxy-3,4-dimethyl-phenyl)-1-methyl-ethylamine | |||
| Other names
3,4-Dimethyl-2,5-dimethoxyamphetamine; 2-(3,4-Dimethyl-2,5-dimethoxyphenyl)-1-methyl-1-aminoethane | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
PubChem CID |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
C13H21NO2 | ||
| Molar mass | 223.316 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
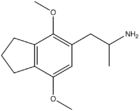
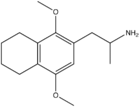
Homologues
G-5
3,6-Dimethoxy-4-(2-aminopropyl)benzonorbornane:[4]
Legality
United Kingdom
This substance is a Class A drug in the Drugs controlled by the UK Misuse of Drugs Act.[6]
References
- Ganesha Entry in PiHKAL
- G-3 entry in PiHKAL
- G-4 entry in PiHKAL
- G-5 entry in PiHKAL
- G-N entry in PiHKAL
- "UK Misuse of Drugs act 2001 Amendment summary". Isomer Design. Retrieved 12 March 2014.