Benzphetamine
Benzphetamine (brand name Didrex) is a substituted amphetamine used short-term along with a doctor-approved, reduced-calorie diet, exercise, and behavioral program for weight loss. It is prescribed for obesity in individuals who have been unable to lose weight through exercise and dieting alone. It is a prodrug to dextroamphetamine and dextromethamphetamine.[2][3][4]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Didrex, Recede |
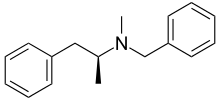
| Other names | N-benzyl-N-methylamphetamine |
| AHFS/Drugs.com | Consumer Drug Information |
| Pregnancy category |
|
| Dependence liability | High[1] |
| Routes of administration | oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 75–99% |
| Elimination half-life | 4-6 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H21N |
| Molar mass | 239.362 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Benzphetamine is an anorectic, primarily promoting weight loss through reduced appetite. Benzphetamine also slightly increases metabolism.
Pharmacology
Benzphetamine is a sympathomimetic amine and is classified as an anorectic. The drug's main function is to reduce appetite, which in turn reduces caloric intake.
Although the mechanism of action of the sympathomimetic appetite suppressants in the treatment of obesity is not fully known, these medications have pharmacological effects similar to those of amphetamines. Amphetamine and related sympathomimetic medications (such as benzphetamine) are thought to stimulate the release of norepinephrine and/or dopamine from storage sites in nerve terminals of the lateral hypothalamic feeding center, thereby producing a decrease in appetite. This release is mediated through the binding of benzphetamine to VMAT2 and inhibiting its function, causing a release of these neurotransmitters into the synaptic cleft through their reuptake transporters. Tachyphylaxis and tolerance have been demonstrated with all drugs of this class.
Contraindications
Benzphetamine is contraindicated in patients with advanced arteriosclerosis, symptomatic cardiovascular disease, moderate to severe hypertension, hyper-thyroidism, known hypersensitivity or idiosyncrasy to sympathomimetic amines, and glaucoma, or who have recently used a MAOI. Benzphetamine should not be given to patients who are in an agitated state or who have a history of drug abuse.[6]
Controlled substance classification
Benzphetamine is unique in its classification as a Schedule III drug in the United States. (Most members of the amphetamine family are classified in the more highly regulated Schedule II.) Benzphetamine is metabolized by the human body into amphetamine and methamphetamine, making it one of a number of drugs to undergo in vivo conversion to a substance of higher addiction and abuse potential.[7]
References
- "Benzphetamine". Toxnet.
- AHC Media, LLC (17 March 2014). Pediatric Trauma Care II: A clinical reference for physicians and nurses caring for the acutely injured child. AHC Media, LLC. pp. 118–. ISBN 978-1-934863-59-6.
- Cody, J. T; Valtier, S (1998). "Detection of amphetamine and methamphetamine following administration of benzphetamine". Journal of Analytical Toxicology. 22 (4): 299–309. doi:10.1093/jat/22.4.299. PMID 9681333.
- Budd, Robert D; Jain, Naresh C (1978). "Short Communication: Metabolism and Excretion of Benzphetamine: Sources of Error in Reporting Results". Journal of Analytical Toxicology. 2 (6): 241. doi:10.1093/jat/2.6.241.
- Woo, Teri. Pharmacotherapeutics for Advanced Practice Nurse Prescribers, 4th Edition. p. 226. ISBN 978-0-8036-3827-3.
- "Benzphetamine". Toxnet.
- Musshoff F (February 2000). "Illegal or legitimate use? Precursor compounds to amphetamine and methamphetamine". Drug Metab. Rev. 32 (1): 15–44. doi:10.1081/DMR-100100562. PMID 10711406.