Bupropion/naltrexone
Bupropion/naltrexone is a combination drug used for weight loss in those that are either obese or overweight with some weight-related illnesses.[1][2] It combines low doses of bupropion and naltrexone. Both drugs have individually shown some evidence of effectiveness in weight loss, and the combination has been shown to have some synergistic effects on weight.[3]
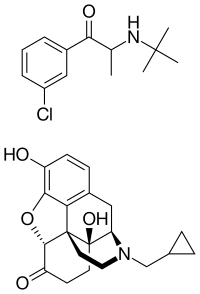 Skeletal structures of bupropion (top) and naltrexone (bottom) | |
| Combination of | |
|---|---|
| Bupropion | Norepinephrine-dopamine reuptake inhibitor and nicotinic acetylcholine receptor antagonist |
| Naltrexone | Opioid receptor antagonist |
| Clinical data | |
| Trade names | Contrave |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| PubChem CID | |
| KEGG | |
| | |
In September 2014, a sustained release formulation of the drug was approved for marketing in the United States under the brand name Contrave.[1] The combination was subsequently approved in the European Union in the spring of 2015, where it is sold under the name Mysimba.[4] It was approved in Canada under the Contrave brand name in 2018.
Medical uses
Bupropion/naltrexone is approved to be used, in conjunction to exercise and dietary changes, in the long-term treatment of adults that are either:[5]
- Obese
- Overweight and have a body mass index of at least 27 kg/m2, and have at least one weight-related comorbidity, like type 2 diabetes or high blood pressure.
Contraindications
The manufacturer recommends against its use in people that have/are:[5]
- History of seizures
- History of an eating disorder such as bulimia nervosa or anorexia nervosa
- Taking opioid pain medicines, taking medicines to stop opioid addiction, or are in opiate withdrawal
- Taking an MAOI or have taken an MAOI in the last 14 days
- Pregnant
- Abruptly stopped using: alcohol, benzodiazepines, barbiturates, or antiepileptic drugs
Adverse effects
The FDA has put a boxed warning onto this medicine because it may affect mood and increase the likelihood of suicide.[5]
Safety and effectiveness in children under the age of 18 has not been studied.[5]
Mechanism of action
Individually, bupropion and naltrexone each target pathways in the central nervous system that influence appetite and energy use.
- Bupropion is a reuptake inhibitor and releasing agent of both norepinephrine and dopamine, and a nicotinic acetylcholine receptor antagonist, and it activates proopiomelanocortin (POMC) neurons in the hypothalamus which give an effect downstream, resulting in loss of appetite and increased energy output. The POMC is regulated by endogenous opioids via opioid-mediated negative feedback.
- Naltrexone by contrast is a pure opioid antagonist, therefore further augmenting bupropion's activation of the POMC.[6]
Combined, bupropion/naltrexone has an effect on the reward pathway that results in reduced food craving.[7] In 2009, Monash University physiologist Michael Cowley was awarded one of Australia's top research honors, the Commonwealth Science Minister's Prize for Life Scientist of the Year, in recognition of his elucidation of these pathways, which led to the development of the combination medication.[8]
History
Orexigen submitted a New Drug Application (NDA) for this drug combination to the FDA On 31 March 2010.[9] Having paid a fee under the Prescription Drug User Fee Act, Orexigen was given a deadline for the FDA to approve or reject the drug of 31 January 2011. On 7 December 2010, an FDA Advisory Committee voted 13-7 for the approval of Contrave, and voted 11-8 for the conduct of a post-marketing cardiovascular outcomes study.[10] Subsequently, on 2 February 2011, the FDA rejected the drug and it was decided that an extremely large-scale study of the long-term cardiovascular effects of Contrave would be needed, before approval could be considered.[11] It was ultimately approved in the United States in the fall of 2014.[1]
In December 2014, the EU's Committee for Medicinal Products for Human Use (CHMP) endorsed the combination for licensure as an obesity medication when used alongside diet and exercise.[12] Approval was granted in late March 2015.[4]
In May 2015, Orexigen ended a safety study of its diet drug earlier than planned, because an independent panel of experts says the drug maker “inappropriately” compromised the trial by prematurely releasing interim data. The early data release reported a reduction in heart attacks that was no longer observed when a more complete view of the data was analyzed.[13]
Marketing and sales
The sustained-release formulation, Contrave, is marketed by Takeda under license from the combination medication's developer, Orexigen Therapeutics.[1] As of 2015, Orexigen received 20% of net sales from Takeda.[14]
At the time of its approval by FDA, Wells Fargo analyst Matthew Andrews estimated that Contrave's U.S. sales would reach approximately US$200,000,000 in 2016, exceeding that of the dominant alternative obesity medications lorcaserin and phentermine/topiramate.[15] Despite being initially impeded by technical issues, the growth in filled prescriptions in the first months after approval was very rapid — substantially exceeding the equivalent early uptake of either of the two alternative medications just cited.[16] The first quarter of sales for Contrave (Q1 2015) showed net sales of US$11,500,000.[14]
Despite having been approved for use in Europe in March 2015, sales of Contrave have not begun as Orexigen has not yet found a marketing partner.[14]
References
- "FDA approves weight-management drug Contrave" (Press release). FDA. 10 September 2014.
- Plodkowski, Raymond A.; Nguyen, Quang; Sundaram, Umasankari; Nguyen, Loida; Chau, Diane L.; St. Jeor, Sachiko (2009). "Bupropion and naltrexone: a review of their use individually and in combination for the treatment of obesity". Expert Opinion on Pharmacotherapy. 10 (6): 1069–1081. doi:10.1517/14656560902775750. PMID 19364254.
- Tek, C (2016). "Naltrexone HCI/bupropion HCI for chronic weight management in obese adults: patient selection and perspectives". Patient Preference and Adherence. 10: 751–9. doi:10.2147/PPA.S84778. PMC 4862388. PMID 27217728.
- Orexigen Therapeutics, Inc. (March 26, 2015). "Orexigen's Mysimba™ Approved in Europe for the Treatment of Obesity". Yahoo! Finance. PR Newswire. Retrieved 28 March 2015.
- "Contrave Prescribing Information". Takeda. Archived from the original on 2014-11-02. Retrieved 2 November 2014.
- Greenway, Frank; Whitehouse, M.J; Guttadauria, Maria; Anderson, James (2008). "Rational Design of a Combination Medication for the Treatment of Obesity". Obesity. 17 (1): 30–39. doi:10.1038/oby.2008.461. PMID 18997675.
- Apovian, Caroline; Aronne, Louis; Rubino, Domenica; Still, Christopher (2013). "A randomized, Phase 3 Trial of Naltrexone SR/Bupropion SR on Weight and Obesity-related Risk Factors (COR-II)". Obesity. 21 (5): 935–943. doi:10.1002/oby.20309. PMC 3739931. PMID 23408728.
- "Obesity expert named Life Scientist of the Year". Monash University. 29 October 2009. Archived from the original on 2 November 2009.
- Orexigen(R) Therapeutics Submits Contrave(R) New Drug Application to FDA for the Treatment of Obesity
- "Press Release". Orexigen Therapeutics, Inc. 2010-12-07. Retrieved 2016-12-29.
- Contrave, Drugs.com
- "Orexigen's weight-loss drug gets thumbs-up from CHMP". FiercePharma. 2014-12-19. Retrieved 2016-12-29.
- Silverman, Ed (2015-05-12). "Orexigen Study for Diet Drug Ends Over Premature Data Disclosure". WSJ. Retrieved 2016-12-29.
- Osborne, Spencer (May 8, 2015). "Orexigen Posts Loss - Revenue Will Be The Story". Seeking Alpha (blog). Retrieved May 9, 2015.
- Grover, Natalie (September 10, 2014). "Reuters More: Reuters Health Long-awaited Diet Pill Gets U.S. Approval Reuters". Business Insider. Reuters. Retrieved March 28, 2015.
- Osborne, Spencer (December 12, 2014). "Contrave Sales Continue To Impress". Seeking Alpha (blog). Retrieved March 28, 2015.