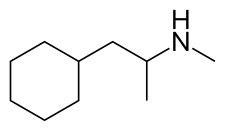
Propylhexedrine
Propylhexedrine, sold under the brand names Benzedrex and Obesin among others, is a nasal decongestant, appetite suppressant, and psychostimulant medication. It is used medicinally for relief of congestion due to colds, allergies and allergic rhinitis and recreationally for its euphoric effects. The effects are similar to those of methamphetamine, though the duration of propylhexedrine is much shorter. Propylhexedrine differs from methamphetamine only in that it has a saturated cyclohexane ring where methamphetamine has a phenyl ring.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Benzedrex, Obesin |
| Other names | Hexahydro-desoxyephedrine; Hexahydro-methamphetamine; Dimethylcyclo-hexaneethanamine; Cycohexyliso-propylmethylamine |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Medical: Intranasal (inhaler) and oral Recreational: Oral and parenteral routes |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 4 ± 1.5 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.673 |
| Chemical and physical data | |
| Formula | C10H21N |
| Molar mass | 155.29 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
| | |
Propylhexedrine is most commonly found in over-the-counter Benzedrex inhalers. Benzedrex was first manufactured by Smith, Kline and French after the Benzedrine inhaler, which contained racemic amphetamine, became unavailable following the placement of amphetamines on the US Schedule II status (highest abuse potential, yet with accepted medicinal uses). Benzedrex is currently manufactured by B.F. Ascher & Co. Inc. Pharmaceuticals.[1]
Propylhexedrine has also seen use in Europe as an appetite suppressant under the trade name Obesin[2] and in the anticonvulsant preparation barbexaclone its S-isomer (levopropylhexedrine or L-propylhexedrine) is bonded with phenobarbital for the purpose of offsetting the barbiturate-induced sedation.[2] Levopropylhexedrine is also used as an anorectic under the brand name Eventin.[3]
Medical use
Propylhexedrine is used to treat acute nasal congestion related to common cold,[1] allergies and hay fever. For nasal congestion, the dosage is listed as four inhalations (two inhalations per nostril) every two hours for adults and children 6–12 years of age. Each inhalation delivers 0.4 to 0.5 milligram (400 to 500 μg) in 800 millilitres of air.[1] Historically, it has also been used for weight loss, typically at doses from 5 to 30 milligrams.[4][5]
Contraindications
Propylhexedrine should not be used if an MAOI has been used in the past 14 days, or is being currently used, as this can lead to a hypertensive crisis. People with cardiovascular disease should not use propylhexedrine.[6]
Additionally, drugs such as stimulants and sympathomimetics should not be taken with propylhexedrine, as this can lead to potentially dangerous spikes in blood pressure and irregular heart rhythms.[7]
There is one case of death where a combination of propylhexedrine, acetaminophen, morphine, promethazine, and kratom was detected. However the study indicates that kratom was not included as one of the causes of death and that propylhexedrine was the principal cause of death.[8]
Pharmacology
Propylhexedrine is a TAAR1 agonist, like amphetamine.[9] Consequently, it reverses the transporters for dopamine, norepinephrine, and serotonin, leading to a release of monoamines from presynaptic vesicles into the synaptic cleft.[9] The increased level of monoamines within the synapse results in increased activity at their respective receptors. Additionally, propylhexedrine appears to inhibit VMAT2, leading to a further increase in the aforementioned monoamines.[9] The pharmacological actions of propylhexedrine are similar to that of structurally similar stimulant phenethylamines, such as amphetamine.[9]
Metabolism
Propylhexedrine undergoes metabolism to form various metabolites including norpropylhexedrine, cyclohexylacetoxime, cis- and trans-4-hydroxypropylhexedrine.[10]
Chemistry
Free base propylhexedrine is a volatile, oily liquid at room temperature. The slow vaporization of this form of the chemical allows it to be administered via inhalation.[11] As an amine, it can easily be protonated to give various ammonium forms, such as propylhexedrine hydrochloride, propylhexedrine citrate, or propylhexedrine acetate, depending on the acid used. These salts are often stable, clear to off-white crystalline powders that readily dissolve in water.[12]
Propylhexedrine is structurally similar to phenylethylamines, with the only structural difference being the substitution of an alicyclic cyclohexyl group for the aromatic phenyl group of phenethylamine. Propylhexedrine is not an amphetamine, nor even a phenethylamine, but instead can be referred to as a cycloalkylamine.
Propylhexedrine is a chiral compound (the α-carbon is chiral), and active ingredient contained in Benzedrex inhalers is racemic (RS)-propylhexedrine as the free base. (S)-Propylhexedrine, also known as levopropylhexedrine, is believed to be the more biologically active isomer of the two.[13] (S)-Propylhexedrine can be synthesized from methamphetamine.[14]
Synthesis
Propylhexedrine can be synthesized starting with cyclohexylacetone in a similar fashion to the phenylacetone synthesis of methamphetamine.[15]
However, more commonly propylhexedrine is prepared by reacting methamphetamine with Adams' catalyst, reducing methamphetamine's aromatic ring to a cyclohexyl moiety.
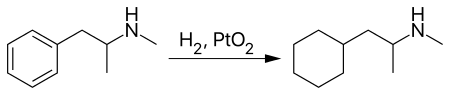
Society and culture
Recreational use
Propylhexedrine is used recreationally in a similar manner to amphetamine, methamphetamine and dextroamphetamine. Users report a high comparable to that of amphetamine and typically inferior to methamphetamine.
Long-term health problems may develop from daily use. Compared to dextroamphetamine, it is a heavier strain on the cardiovascular system, and the risk of heart damage increases when used daily.
Effects
Propylhexedrine has sympathomimetic, adrenergic, vasoconstrictive and psychostimulant effects when taken above the medical dosage. Effects include increased sweating, talkativeness, mydriasis, emotional lability, anorexia, tachycardia, palpitations, dry mouth, bruxism, anxiety, euphoria or dysphoria, increased aggressiveness, paranoia, headache, dizziness, psychosis, slurred or impaired speech, rarely convulsions and serious heart problems.[17] Propylhexedrine can also cause swelling, dryness and irritation of mucous membranes.[18]
Injection risks
While propylhexedrine is limited in a number of administration routes, attempts to extract the drug from the nasal inhaler and then inject it have been reported. Recreational use by intravenous injection (IV) is dangerous and could result in serious bodily harm or death. IV use of propylhexedrine is known to cause mild side-effects such as transient diplopia as well as some serious (and potentially fatal) effects such as brainstem dysfunction, and deaths have been recorded in the medical literature. Typically, recorded cases of IV use are prepared by forming propylhexedrine HCl in a solution with hydrochloric acid, the solution is then heated to evaporate and the resulting crystals are dissolved in water for injection.[19][20][17]
See also
References
- "Benzedrex Inhaler Nasal Decongestant Inhaler". B.F. Ascher & Company, inc. Retrieved 27 March 2013.
- Wesson DR (June 1986). "Propylhexdrine". Drug and Alcohol Dependence. 17 (2–3): 273–8. doi:10.1016/0376-8716(86)90013-X. PMID 2874970.
- "Eventin". Drugs.com. Retrieved 27 March 2013.
- Docherty, J R (1 June 2008). "Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA)". British Journal of Pharmacology. 154 (3): 606–622. doi:10.1038/bjp.2008.124. PMC 2439527. PMID 18500382.
- "Obesin Dosage, Interactions". Retrieved 23 October 2017.
- Propylhexedrine Contraindications - Medscape
- Safety of mixing stimulants with medications - NIDA
- Holler, J. M.; Vorce, S. P.; McDonough-Bender, P. C.; Magluilo, J.; Solomon, C. J.; Levine, B. (2011). "A Drug Toxicity Death Involving Propylhexedrine and Mitragynine" (PDF). Journal of Analytical Toxicology. 35 (1): 54–9. doi:10.1093/anatox/35.1.54. PMID 21219704.
- Propylhexedrine's DrugBank Entry
- Midha, KK; Beckett, AH; Saunders, A (1974). "Identification of the Major Metabolites of Propylhexedrine in vivo (in man) and in vitro (in guinea pig and rabbit)". Xenobiotica. 4 (10): 627–635. doi:10.1080/00498257409169765. PMID 4428789.
- Nasal Inhaler, US 4095596
- Mancusi-Ungaro, H. R. Jr. M.D.; Decker, W. J.; Forshan, V. R. D. O.; Blackwell, S. J. M.D.; Lewis, S. R. M.D. (1983). "Tissue Injuries Associated With Parenteral Propylhexedrine Abuse". Journal of Trauma-Injury Infection & Critical Care. 23 (7): 650. doi:10.1097/00005373-198307000-00114.CS1 maint: multiple names: authors list (link)
- A. M. Lands; V. L. Nash; H. R. Granger; B. L. Dertinger (1947). "The Pharmacologic Activity of N-Methyl-β-cyclohexylisopropylamine Hydrochloride". Journal of Pharmacology and Experimental Therapeutics. 89 (3): 382–385. PMID 20295519.
- Textbook of organic medicinal and pharmaceutical chemistry, Charles Owens Wilson, Ole Gisvold, Robert F. Doerge, page 491
- Merck Index 7761 - Kleeman & Engel p.774 OCDS Vol.1 p.37 (1977) I.N. p.817
- Zenitz, BL; Macks, EB; Moore, ML (May 1947). "Preparation of Some Primary and Secondary β-Cyclohexylalkylamines". Journal of the American Chemical Society. 69 (5): 1117–21. doi:10.1021/ja01197a039. PMID 20240502.
- Fornazzari, L; Carlen, PL; Kapur, BM (1986). "Intravenous Abuse of Propylhexedrine (Benzedrex®) and the Risk of Brainstem Dysfunction in Young Adults". Canadian Journal of Neurological Sciences. 13 (4): 337–339. doi:10.1017/S0317167100036696. PMID 2877725.
- Safety Data Sheet - Cayman Chemical
- "Proposed Rules". Federal Register. 50 (10): 2226–2227.
- Prince v. Ascher, 90 P.3d 1020 (2004).