Rimonabant
Rimonabant (also known as SR141716; trade names Acomplia, Zimulti)[3] is an anorectic antiobesity drug that was first approved in Europe in 2006 but was withdrawn worldwide in 2008 due to serious psychiatric side effects; it was never approved in the United States.[1][2] Rimonabant is an inverse agonist for the cannabinoid receptor CB1 and was the first drug approved in that class.[4][5]
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Undetermined |
| Protein binding | Nearly 100% |
| Metabolism | Hepatic, CYP3A4 involved |
| Elimination half-life | Variable: 6 to 9 days with normal BMI 16 days if BMI >30 |
| Excretion | Fecal (86%) and renal (3%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.978 |
| Chemical and physical data | |
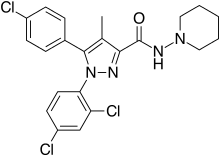
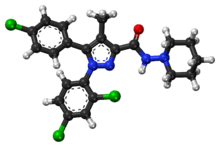
| Formula | C22H21Cl3N4O |
| Molar mass | 463.79 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
History
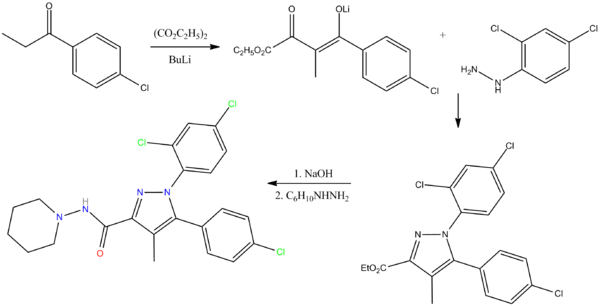
Rimonabant is a selective CB1 receptor blocker and was discovered and developed by Sanofi-Aventis;[6]
On 21 June 2006, the European Commission approved the sale of rimonabant in the then-25-member European Union as a prescription drug for use in conjunction with diet and exercise for patients with a body mass index (BMI) greater than 30 kg/m2, or patients with a BMI greater than 27 kg/m2 with associated risk factors, such as type 2 diabetes or dyslipidaemia.[7] It was first in its class to be approved anywhere in the world.[5]
Rimonabant was submitted to the Food and Drug Administration (FDA) for approval in the United States in 2005; in 2007, the FDA's Endocrine and Metabolic Drugs Advisory Committee (EMDAC) concluded that Sanofi-Aventis failed to demonstrate the safety of rimonabant and voted against recommending the anti-obesity treatment for approval[8] and two weeks later the company withdrew the application.[9]
The drug was approved in Brazil in April 2007.[2]
In October 2008, the European Medicines Agency recommended the suspension of Acomplia after the Committee for Medicinal Products for Human Use (CHMP) had determined that the risks of Acomplia outweighed its benefits due to the risk of serious psychiatric problems, including suicide.[10] In November 2008 an advisory committee in Brazil recommended suspension as well, and that month Sanofi-Aventis suspended sale of the drug worldwide.[2] The EMA approval was withdrawn in January 2009.[11][12] In 2009 India prohibited the manufacture and sale of the drug.[13]
Adverse effects
Data from clinical trials submitted to regulatory authorities showed that rimonabant caused depressive disorders or mood alterations in up to 10% of subjects and suicidal ideation in around 1%, and in Europe it was contraindicated for people with any psychiatric disorder, including depressed or suicidal individuals.[7]
Additionally, nausea and upper respiratory tract infections were very common (occurring in more than 10% of people) adverse effects; common adverse effects (occurring in between 1% and 10% of people) included gastroenteritis, anxiety, irritability, insomnia and other sleep disorders, hot flushes, diarrhea, vomiting, dry or itchy skin, tendonitis, muscle cramps and spasms, fatigue, flu-like symptoms, and increased risk of falling.
The FDA's advisory committee raised concerns that based on animal data, it appeared that the therapeutic window with regard to CNS toxicity, and specifically seizures was almost nonexistent; the therapeutic dose and the dose that caused seizures in animals appeared to be the same.[2][14][15]
When the EMA reviewed postmarketing surveillance data, it found that the risk of psychiatric disorders in people taking rimonabant was doubled.[2]
Research
Along with the clinical trials in obesity that generated the data submitted to regulatory authorities,[17] rimonabant was also studied in clinical trials as a potential treatment for other conditions,[2] including diabetes, atherosclerosis, and smoking cessation.[18][19]
References
- Sam, AH; Salem, V; Ghatei, MA (2011). "Rimonabant: From RIO to Ban". Journal of Obesity. 2011: 432607. doi:10.1155/2011/432607. PMC 3136184. PMID 21773005.
- Moreira, FA; Crippa, JA (June 2009). "The psychiatric side-effects of rimonabant". Revista brasileira de psiquiatria (Sao Paulo, Brazil : 1999). 31 (2): 145–53. doi:10.1590/s1516-44462009000200012. PMID 19578688.
- "Rimonabant". AdisInsight. Retrieved 21 February 2017.
- Fong TM, Heymsfield SB (September 2009). "Cannabinoid-1 receptor inverse agonists: current understanding of mechanism of action and unanswered questions". Int J Obes (Lond). 33 (9): 947–55. doi:10.1038/ijo.2009.132. PMID 19597516.
- "European Approval Comes Early for Sanofi-Aventis' Acomplia". IHS. June 23, 2006.
- Barth, F.; Rinaldi-Carmona, M. (1999), "The Development of Cannabinoid Antagonists", Current Medicinal Chemistry, 6 (8): 745–755, PMID 10469889
- "Acomplia EPAR" (PDF). EMA. January 30, 2009. From EMA index page
- Saul, Stephanie (14 June 2007). "F.D.A. Panel Rejects Drug for Obesity". The New York Times.
- "Sanofi-Aventis Drops Application for Drug". The New York Times. 30 June 2007.
- "The European Medicines Agency recommends suspension of the marketing authorisation of Acomplia". European Medicines Agency. 23 October 2008. Retrieved 18 January 2016.
- "Anti-obesity drug use suspended". BBC News. 23 October 2008. Retrieved 4 March 2010.
- "Public Statement on Acomplia (rimonabant) Withdrawal of the Marketing Authorisation in the European Union" (PDF). European Medicines Agency. 30 January 2009. Retrieved 18 January 2016.
- "Drugs banned in India". Central Drugs Standard Control Organization, Dte.GHS, Ministry of Health and Family Welfare, Government of India. Retrieved 2013-09-17.
- "FDA Briefing Document NDA 21-888 Zimulti (rimonabant) Tablets, 20" (PDF). FDA. June 13, 2007.
- Davis-Bruno, Karen (June 13, 2007). "Nonclinical Overview: CNS Toxicity with Rimonabant". FDA, Division of Metabolism & Endocrinology Products.
- Yoshioka, T.; et al. (1989). "Studies on hindered phenols and analogs. 1. Hypolipidemic and hypoglycemic agents with ability to inhibit lipid peroxidation". Journal of Medicinal Chemistry. 32 (2): 421–8. doi:10.1021/jm00122a022. PMID 2913302.
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J (Feb 2006). "Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial". JAMA. 295 (7): 761–75. doi:10.1001/jama.295.7.761. PMID 16478899.
- Cahill, K; Ussher, MH (16 March 2011). "Cannabinoid type 1 receptor antagonists for smoking cessation". The Cochrane Database of Systematic Reviews (3): CD005353. doi:10.1002/14651858.CD005353.pub4. PMC 6486173. PMID 21412887.
- Maldonado R, Valverde O, Berrendero F (2006). "Involvement of the endocannabinoid system in drug addiction". Trends Neurosci. 29 (4): 225–32. doi:10.1016/j.tins.2006.01.008. PMID 16483675.