LY-2183240
LY-2183240 is a drug which acts both as a potent inhibitor of the reuptake of the endocannabinoid anandamide and as an inhibitor of fatty acid amide hydrolase (FAAH), the primary enzyme responsible for degrading anandamide. This leads to markedly elevated anandamide levels in the brain, and LY-2183240 has been shown to produce both analgesic and anxiolytic effects in animal models.[1][2][3] While LY-2183240 is a potent inhibitor of FAAH, it has relatively poor selectivity and also inhibits several other enzyme side targets.[4] Consequently, it was never developed for clinical use, though it remains widely used in research, and has also been sold as a designer drug.[5]
 | |
| Names | |
|---|---|
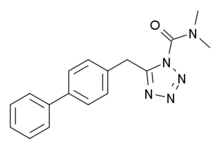
| IUPAC name
N,N-dimethyl-5-[(4-biphenyl)methyl]tetrazole-1-carboxamide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.189.657 |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C17H17N5O |
| Molar mass | 307.349 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
See also
- Endocannabinoid reuptake inhibitor
- FAAH inhibitor
References
- Dickason-Chesterfield AK, Kidd SR, Moore SA, Schaus JM, Liu B, Nomikos GG, Felder CC. Pharmacological characterization of endocannabinoid transport and fatty acid amide hydrolase inhibitors. Cellular and Molecular Neurobiology. 2006 Jul-Aug;26(4-6):407-23. PMID 16736384
- Maione S, Morera E, Marabese I, Ligresti A, Luongo L, Ortar G, Di Marzo V. Antinociceptive effects of tetrazole inhibitors of endocannabinoid inactivation: cannabinoid and non-cannabinoid receptor-mediated mechanisms. British Journal of Pharmacology. 2008 Nov;155(5):775-82. PMID 18660824
- Powers MS, Barrenha GD, Mlinac NS, Barker EL, Chester JA. Effects of the novel endocannabinoid uptake inhibitor, LY2183240, on fear-potentiated startle and alcohol-seeking behaviors in mice selectively bred for high alcohol preference. Psychopharmacology. 2010 Dec;212(4):571-83. PMID 20838777
- Alexander JP, Cravatt BF. The putative endocannabinoid transport blocker LY2183240 is a potent inhibitor of FAAH and several other brain serine hydrolases. Journal of the American Chemical Society. 2006 Aug 2;128(30):9699-704. PMID 16866524
- Uchiyama N, Matsuda S, Kawamura M, Shimokawa Y, Kikura-Hanajiri R, Aritake K, Urade Y, Goda Y. Characterization of four new designer drugs, 5-chloro-NNEI, NNEI indazole analog, α-PHPP and α-POP, with 11 newly distributed designer drugs in illegal products. Forensic Sci Int. 2014 Oct;243:1-13. PMID 24769262 doi:10.1016/j.forsciint.2014.03.013.
Further reading
Moore, S. A.; Nomikos, G. G.; Dickason-Chesterfield, A. K.; Schober, D. A.; Schaus, J. M.; Ying, B. P.; Xu, Y. C.; Phebus, L; Simmons, R. M.; Li, D; Iyengar, S; Felder, C. C. (2005). "Identification of a high-affinity binding site involved in the transport of endocannabinoids". Proceedings of the National Academy of Sciences. 102 (49): 17852–7. doi:10.1073/pnas.0507470102. PMC 1295594. PMID 16314570.