Mitragynine
Mitragynine is an indole-based alkaloid and the most abundant active alkaloid in the Southeast Asian plant Mitragyna speciosa, commonly known as kratom.[2] Mitragynine accounts for approximately 60% of the total alkaloid content in kratom leaves from which mitragynine preparations are derived.[3] Such preparations are orally consumed and typically involve dried kratom leaves which are brewed into tea[2][3] or ground and placed into capsules.[3] Mitragynine consumption for medicinal and recreation purposes dates back centuries, although early use was primarily limited to Southeast Asian countries such as Thailand where the plant grows indigenously.[4] Still today, The kratom leaf is reportedly the most commonly used illicit drug in Thailand where it became listed as a controlled substance in 1943.[5] [6] Recently, mitragynine use has spread throughout Europe and the Americas as both a recreational and medicinal drug.[6] While research into the effects of kratom have only recently begun to emerge, investigations on the purified active compound mitragynine are even less common.
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H30N2O4 |
| Molar mass | 398.5 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 102–106 °C [1] |
SMILES
| |
InChI
| |
Uses
Medical
*Medical uses below have not been evaluated by the FDA
Anti-nociception
Mitragynine, along with additional alkaloids found in the kratom plant have been used to mitigate pain for over a century.[4][6][7] [2] In Southeast Asia, the consumption of mitragynine from whole leaf kratom preparations is common amongst laborers who utilized the antinociceptive and mildly stimulating effects of mitragynine to increase endurance and ease pain while working.[7][2] In animal models, alkaloid containing extracts of kratom have been shown to induce naloxone reversible antinociceptive effects as indicated in hotplate and tail-flick assays.[8] In a comparative analysis of mitragynine and traditional opioids agonists such as morphine and oxycodone, mitragynine appeared to have an antinociceptive effect comparable to oxycodone in rats.[8]
Opioid withdrawal
As early as the 19th century, mitragynine, ingested in the form of kratom leaf or tea, was reportedly used for the treatment of opium addiction and withdrawal.[4] [2] Still today, many users of mitragynine list opioid replacement and opioid withdrawal as their primary motivations for ingesting kratom. In an online survey of approximately 8,000 kratom users, nearly 50% indicated that their use of kratom resulted in the reduction or discontinuation of other opioids. [7] An additional study included in the same systematic review found that in 136 Malaysian kratom users, 90% were using the plant as a substitute for other opioids and 84% found that the effects of kratom helped with opioid withdrawal.[7] Animal models of opioid withdrawals suggest mitragynine can suppress and ameliorate withdrawal from other opioid agonists. For example, mitragynine was shown to ameliorate the withdrawal symptoms induced by chronic administration of morphine in zebra fish.[9]
Recreational
Mitragynine and its metabolite 7-hydroxymitragynine are thought to underlie the intoxicating effects of kratom.[3][2] Consumption of dried kratom leaves yields biphasic responses depending on the dose consumed.[3][2][4] At low doses, the plant is reported to induce a mild stimulating effect, while larger doses are reported to produce sedation and nociception typical of opioids.[3][4][2] The concentration of mitragynine as well as other alkaloids in kratom have been found to vary between different strains of the plant, thus contributing to strain specific effects as well.[3] Kratom extracts containing concentrated levels of mitragynine are often mixed with other easily attainable psychoactive compounds such as cough medicine to potentiate the effects.[3] The abuse potential of mitragynine and other kratom alkaloids has been documented in animal studies such as conditioned place preference test (CPP) which indicated a distinct rewarding effect of 7-hydroxymitragynine.[8]
Dependence and withdrawal
Due to its activity on opioid receptors, mitragynine itself can result in dependence and lead to withdrawal symptoms when discontinued. Regular users report withdrawal symptoms comparable to that of other opioids following the discontinuation of kratom. A 2014 study which included 1118 male kratom users indicated that more than half of the regular users (67% of total subjects) experienced withdrawal when attempting to discontinue kratom with symptoms that included pain, muscle spasms, and insomnia.[7] In a study following 239 male kratom users in Malaysia consuming between 40 and 240 mg of mitragynine per day, 89% indicated a previous attempt of discontinuing kratom consumption which resulted in withdrawal symptoms ranging from mild (65% of subjects) to moderate/severe (35% of subjects).[10] In the same study, withdrawal symptoms ranged from physical symptoms such as nausea, diarrhea, and muscle spasms to psychological symptoms such as restlessness, anxiety, and anger but lasted less than 3 days for most subjects.[10] However, the results from this study may be obfuscated by the occasional addition of other substances in the mitragynine preparation such as dextromethorphan and benzodiazepines, which could contribute to the withdrawal symptoms[10]. In an animal study, mitragynine withdrawal symptoms were observed following 14 days of mitragynine i.p. injections in mice and included displays of anxiety, teeth chattering, and piloerection, all of which are characteristic signs of opioid withdrawal in mice and are comparable to morphine withdrawal symptoms[10].
Pharmacology
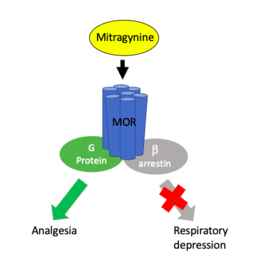
Pharmacodynamics
Mitragynine acts on a variety of receptors in the CNS, most notability the mu, delta, and kappa opioid receptors[11]. The nature of mitragynines' interaction with opioid receptors has yet to be fully classified with some reports suggesting partial agonist activity at the mu opioid receptor[4] [11] [12] and others suggesting full agonist activity.[3] Additionally, mitragynine is known to interact with delta and kappa opioid receptors as well, but these interactions remain ambiguous with some reports indicated mitragynine as a delta and kappa competitive antagonist[11] and others as a full agonist of these receptors.[3] In either case, mitragynine is reported to have lower affinity to delta and kappa receptors compared to mu receptors.[2] Mitragynine is also known to interact with dopamine D2, adenosine, serotonin, and alpha-2 adrenergic receptors, though the significance of these interactions is not fully understood.[11][3] Additionally, several reports of mitragynine pharmacology indicate potential biased agonism activity favoring G protein signaling pathways independent of beta - arrestin recruitment [11][6][4][12], a primary component in opioid induced respiratory depression. [11]
Pharmacokinetics
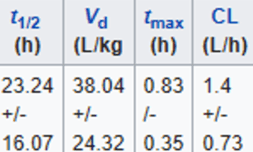
Pharmacokinetic analysis have largely taken place in live rodents as well as rodent and human microsomes.[11] Owing to the heterogeneity of analysis and paucity of human experiments conducted thus far, the pharmacokinetic profile of mitragynine is not complete.[11] However, initial pharmacokinetic studies in humans have yielded preliminary information.[4] [11] In a study of 10 healthy volunteers taking orally administered mitragynine from whole leaf preparations, mitragynine appeared to have a much longer half-life than typical opioid agonists (7-39 hours) and reached peak plasma concentration within 1 hour of administration.[3]
Metabolism
.png)
| CYP | 1A2 | 3A4 | 2D6 |
|---|---|---|---|
| IC50 (ug/ml) | 39 (6) | 0.78 (6) | 3.6 (3), 0.636(6) |
Mitragynine is primarily metabolized in the liver producing many metabolites during both phase I and phase II.[4]
Phase I
During phase I metabolism, mitragynine undergoes hydrolysis of the methylester group on C16 as well as o-demethylation of both methoxy groups on positions 9 and 17.[13] [2] Following this step, oxidation and reduction reactions convert aldehyde intermediates into alcohols and carboxylic acids. [2] P450 metabolic enzymes are known to facilitate the phase I metabolism of mitragynine which reportedly has an inhibitory effect on multiple P450 enzymes, raising the possibility of adverse drug interactions.[14][2] [11]
Phase II
During phase II metabolism, phase I metabolites undergo glucuronidation and sulfation to form multiple glucuronide and sulfate conjugates, that are eliminated via urine.[11] [2]
Toxicology
Mitragynine toxicity in humans is largely unknown as studies examining mitragynine toxicity have thus far used animals which seem to have significant species specific differences in mitragynine tolerance.[3] However, mitragynine toxicity in humans is rarely reported although specific examples of seizures and liver toxicity in kratom consumers have been reported[15][16]. Due to P450 enzyme inhibition, the combination of mitragynine with other drugs poses a major concern regarding adverse reactions to mitragynine.[14][2][3][11] As such, fatalities involving mitragynine tend to involve additional drugs including other opioids and cough suppressants.[3] Post mortem toxicology screens indicate a wide range of mitragynine blood concentrations ranging from 10mcg/L to 4800mcg/L, making it difficult to calculate what constitutes a toxic dose.[16] Such variations in blood concentrations are suggested to result from differences in the toxicology assays used and how long after overdose the assays were conducted.[16]
Legality
In the United States, kratom and its active ingredients are not scheduled under DEA guidelines. Despite the current legal status of the plant and its constituents, the legality of kratom has been turbulent over the past few years. In august 2016 The DEA issued a report of intent stating that mitragynine and 7-hydroxymitragynine would undergo emergency scheduling and be placed under schedule 1 classification until further notice, making kratom strictly illegal and thus hindering research on its active constituents.[17] [6] Shortly following this report of intent, the DEA faced significant public and administrative opposition in the form of a white house petition signed by 140,000 citizens and a letter to the DEA administrator backed by 51 members of the House of Representatives resisting the proposed scheduling.[17] This opposition lead the DEA to withdraw its report of intent in October of 2016, allowing for unencumbered research into the potential benefits and health risks associated with mitragynine and other alkaloids in the kratom plant.[17] [6] Currently, kratom and its active constituents are unscheduled and legally sold in stores and online in the United States.
Research limitations
Inconsistencies in dosing, purity, and concomitant drug use makes evaluating the effects of mitragynine in humans difficult. Conversely, animal studies control for such variability but offer limited translatable information relevant to humans.[11] Experimental limitations aside, mitragynine has been found to interact with a variety of receptors, although the nature and extent of receptor interactions has yet to be fully characterized. [3] Additionally, the toxicity of mitragynine and associated kratom alkaloids have yet to be fully determined in humans, nor has the risk of overdose.[16] While kratom and its alkaloids are currently legal, future studies are necessary to assess safety and potential therapeutic utility.
References
- "Kratom profile (chemistry, effects, other names, origin, mode of use, other names, medical use, control status)". www.emcdda.europa.eu.
- Hassan, Zurina (2013). "From kratom to mitragynine and its derivatives: physiological and behavioral effects related to use, abuse, and addiction". Neuroscience and Behavioral Reviews. 37: 138–151 – via Elsevier.
- Kimheang, Warner (2016). "The pharmacology and toxicology of kratom: from traditional herb to drug of abuse". int J legal Med. 130: 127–138.
- Veltri, Charles (2019). "Current perspectives on the impact of kratom use". Substance Abuse and Rehabilitation. 10: 23–31.
- Tanguay, Pascal (2011). "Kratom in Thailand". International Drug Policy Consortium. 14: 1–16.
- Prozialeck, Walter (2012). "Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects". JAOA. 112: 792–799.
- Swogger, Marc (2018). "Kratom use and mental health: A systematic review". Drug and Alcohol Dependence. 183: 143–140 – via Elsevier.
- Suhaimi, Farah (2016). "Neurobiology of Kratom and its main alkaloid mitragynine". Brain Research Bulletin.
- White, C (2018). "Pharmacologic and clinical assessment of kratom". AM J HEALTH-SYST PHARM. 75: 261–267.
- White, C (2018). "Pharmacologic and clinical assessment of kratom". AM J HEALTH-SYST PHARM. 75: 261–267.
- Ya, Kimheang (2019). "Pharmacokinetics of mitragynine, a major analgesic alkaloid in kratom (Mitragyna speciosa): A systematic review". Asian Journal of Psychiatry. 43: 73–82 – via Science Direct.
- Eldridge, Whitney (2019). "Kratom: An Opioid-like Herbal Supplement Pediatricians Should Know About" (PDF). Journal of Pediatrics and Pediatric Medicine. 3: 1–5.
- "EMCDDA | Kratom profile (chemistry, effects, other names, origin, mode of use, other names, medical use, control status)". www.emcdda.europa.eu. Retrieved 2019-11-18.
- Ulbricht, Cathrine (2013). "An evidence - based systematic review of kratom (mitragyna speciosa) by the natural standard research collaboration". Journal of Dietary Supplements. 10: 152–170.
- Fluyau, Dimy (2017). "Biochemical Benefits, Diagnosis, and Clinical Risks Evaluation of Kratom". Frontiers in psychiatry.
- Alsarraf, Emad (2019). "Kratom from Head to Toe—Case Reviews of Adverse Events and Toxicities". Current Emergency and Hospital Medicine Reports. 7: 141–168.
- Griffin, O (2017). "The scheduling of kratom and selective use of data". Journal of Psychoactive Drugs. 13: 1–7.