Cannabigerol
Cannabigerol (CBG) is one of more than 120 identified cannabinoid compounds found in the plant genus Cannabis.[1][2] Cannabigerol is the non-acidic form of cannabigerolic acid, the parent molecule from which other cannabinoids are synthesized. Cannabigerol is a minor constituent of cannabis.[3] During growth, most of the cannabigerol is converted into other cannabinoids, primarily tetrahydrocannabinol (THC) or cannabidiol (CBD), leaving about 1% cannabigerol in the plant.[4]
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H32O2 |
| Molar mass | 316.485 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Biosynthesis
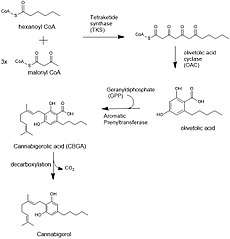
The biosynthesis of cannabigerol begins by loading hexanoyl-CoA onto a polyketide synthase assembly protein and subsequent condensation with three molecules of malonyl-CoA.[5] This polyketide is cyclized to olivetolic acid via olivetolic acid cyclase, and then prenylated with a ten carbon isoprenoid precursor, geranyl pyrophosphate, using an aromatic prenyltransferase enzyme, geranyl-pyrophosphate—olivetolic acid geranyltransferase, to biosynthesize cannabigerolic acid, which can then be decarboxylated to yield cannabigerol.[3][6]
Research
As of 2019, no clinical research has been conducted to test the specific effects of cannabigerol in humans.[7]
Cannabigerol is under laboratory research to determine its pharmacological properties and potential effects in disease conditions.[7][8] Contrary to the major psychoactive cannabinoid THC, cannabigerol antagonizes CB1 receptors and is both an alpha2-adrenoceptor agonist and moderate 5HT1A receptor antagonist.[7][9] Cannabigerol displays CB1 and CB2 binding affinity.[7][8] Additionally, cannabigerol has been evaluated in laboratory models of colitis.[10]
Legal status
Cannabigerol is not scheduled by the UN Convention on Psychotropic Substances. In the United States, it is not a controlled substance under the Controlled Substances Act as long as it is not produced from the controlled parts of the cannabis plant.[11]
See also
References
- Elsohly, M. A; Radwan, M. M; Gul, W; Chandra, S; Galal, A (2017). Phytochemistry of Cannabis sativa L. Progress in the Chemistry of Organic Natural Products. 103. pp. 1–36. doi:10.1007/978-3-319-45541-9_1. ISBN 978-3-319-45539-6. PMID 28120229.
- Turner, S. E; Williams, C. M; Iversen, L; Whalley, B. J (2017). Phytocannabinoids. Progress in the Chemistry of Organic Natural Products. 103. pp. 61–101. doi:10.1007/978-3-319-45541-9_3. ISBN 978-3-319-45539-6. PMID 28120231.
- Morales, P; Reggio, P. H; Jagerovic, N (2017). "An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol". Frontiers in Pharmacology. 8: 422. doi:10.3389/fphar.2017.00422. PMC 5487438. PMID 28701957.
- Aizpurua-Olaizola, Oier; Soydaner, Umut; Öztürk, Ekin; Schibano, Daniele; Simsir, Yilmaz; Navarro, Patricia; Etxebarria, Nestor; Usobiaga, Aresatz (2016-02-26). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–331. doi:10.1021/acs.jnatprod.5b00949. ISSN 0163-3864. PMID 26836472.
- Page, Jonathan; et al. (2012). "Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides". PNAS. 109 (31): 12811–6. doi:10.1073/pnas.1200330109. PMC 3411943. PMID 22802619.
- Meinhart H, Zenk; et al. (1998). "Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol". FEBS Letters. 427 (2): 283–285. doi:10.1016/s0014-5793(98)00450-5. PMID 9607329.
- "Cannabigerol; ID 5315659". PubChem, National Library of Medicine, US National Institutes of Health. 11 May 2019. Retrieved 12 May 2019.
- Morales, P; Hurst, D. P; Reggio, P. H (2017). Molecular targets of the phytocannabinoids - A complex picture. Progress in the Chemistry of Organic Natural Products. 103. pp. 103–131. doi:10.1007/978-3-319-45541-9_4. ISBN 978-3-319-45539-6. PMC 5345356. PMID 28120232.
- Cascio, MG (2010). "Evidence that the plant cannabinoid cannabigerol is a highly potent alpha2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist". British Journal of Pharmacology. 159: 129–41. doi:10.1111/j.1476-5381.2009.00515.x. PMC 2823359. PMID 20002104.
- Couch, D. G; Maudslay, H; Doleman, B; Lund, J. N; O'Sullivan, S. E (2018). "The use of cannabinoids in colitis: A systematic review and meta-analysis" (PDF). Inflammatory Bowel Diseases. 24 (4): 680–697. doi:10.1093/ibd/izy014. PMID 29562280.
- "USC > Title 21 > Chapter 13 > Subchapter I > Part A > § 802. Definitions: (16)" (PDF). Government Publishing Office - US Code. 2016.