Meclofenamic acid
Meclofenamic acid (meclofenamate sodium, brand Meclomen) is a drug used for joint, muscular pain, arthritis and dysmenorrhea.[1] It is a member of the anthranilic acid derivatives (or fenamate) class of NSAID drugs and was approved by the FDA in 1980.[2] Like other members of the class, it is a COX inhibitor and prevents formation of prostaglandins.[3]
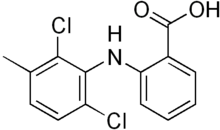 | |
| Clinical data | |
|---|---|
| Trade names | Meclomen |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.382 |
| Chemical and physical data | |
| Formula | C14H11Cl2NO2 |
| Molar mass | 296.14864 g/mol g·mol−1 |
Scientists led by Claude Winder from Parke-Davis invented meclofenamate sodium in 1964, along with fellow members of the class, mefenamic acid in 1961 and flufenamic acid in 1963.[4]:718
Patents on the drug expired in 1985[5]:295 and several generics were introduced in the US, but as of July 2015 only Mylan still sold it.[6][7]
It is not widely used in humans as it has a high rate (30-60%) rate of gastrointestinal side effects.[8]:310 As of 2015 the cost for a typical course of medication in the United States is 50 to US$100.[9]
Use in horses
Meclofenamic acid is sold under the trade name "Arquel" for use in horses, and is administered as an oral granule form at a dose of 2.2 mg/kg/day.[10] It has a relatively slow onset of action, taking 36–48 hours for full effect,[11] and is most useful for treatment of chronic musculoskeletal disease.[12] It has been found to be beneficial for the treatment of navicular syndrome, laminitis, and osteoarthritis,[11] in some cases having a more profound effect than the commonly used NSAID phenylbutazone.[13] However, due to cost, it is not routinely used in practice. Toxicity due to excessive dosage is similar to that of phenylbutazone, including depression, anorexia, weight loss, edema, diarrhea, oral ulceration, and decreased hematocrit.[13]
References
- "meclofenamate, Meclomen: Drug Facts, Side Effects and Dosing". medicinenet.com.
- FDA Meclomen page at FDA Page accessed July 3, 2015
- NIH LiverTox Database Mefenamic Acid Last updated June 23, 2015. Page accessed July 3, 2015
- Whitehouse M. Drugs to Treat Inflammation: A Historical Overview. pp 707-729 in Frontiers in Medicinal Chemistry , Volume 4. Eds Rahman A, et al. Bentham Science Publishers, 2009 ISBN 978-1-60805-207-3
- United States. Congress. Office of Technology Assessment Pharmaceutical R & D: Costs, Risks & Rewards DIANE Publishing, 1993 ISBN 978-0-7881-0468-8
- FDA Meclofenamate sodium ANDAs at FDA Page accessed July 3, 2015
- FDA Mylan label for meclofenamate sodium Revised: October 2013, Accessed July 3, 2015
- Jeffrey K. Aronson. Meyler's Side Effects of Analgesics and Anti-inflammatory Drugs. Elsevier, 2009 ISBN 978-0-08-093294-1
- Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 9. ISBN 9781284057560.
- McIlwraith CW, Frisbie DD, Kawcak CE. Nonsteroidal Anti-Inflammatory Drugs. Proc. AAEP 2001 (47): 182-187.
- Cotter GH, Riley WF, Beck CC, Coppock RW. Arquel (Cl- 1583). A new nonsteroidal anti-inflammatory drug for horses, in Proceedings. Am Assoc Equine Practnr 1973;19: 81–90.
- Snow DH, Baxter P, Whiting B. The pharmacokinetics of meclofenamic acid in the horse. J Vet Pharmacol Ther 1981; 4:147–156.
- Lees P, Higgins AJ. Clinical pharmacology in therapeutic uses of non-steroidal anti-inflammatory drugs in the horse. Equine Vet J 1985;17:83–96.