Glimepiride
Glimepiride, sold under the trade name Amaryl among others, is a medication used to treat diabetes mellitus type 2.[1][2] It is less preferred than metformin.[1] Use is recommended together with diet and exercise.[1] It is taken by mouth.[1] Glimepiride takes up to three hours for maximum effect and lasts for about a day.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Amaryl, other |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696016 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Protein binding | >99.5% |
| Metabolism | Complete Liver (1st stage through CYP2C9) |
| Elimination half-life | 5–8 hours |
| Excretion | Urine (~60%), feces (~40%) |
| Identifiers | |
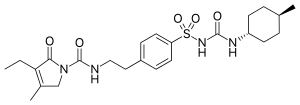
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.170.771 |
| Chemical and physical data | |
| Formula | C24H34N4O5S |
| Molar mass | 490.617 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 207 °C (405 °F) |
SMILES
| |
InChI
| |
| | |
Common side effects include headache, nausea, and dizziness.[1] Serious side effects may include low blood sugar.[1] Use during pregnancy and breastfeeding is not recommended.[3] It works mainly by increasing the amount of insulin released from the pancreas.[1] It is classified as a second-generation sulfonylurea.[4]
Glimepiride was patented in 1979 and approved for medical use in 1995.[5] It is available as a generic medication.[2] A month supply in the United Kingdom costs the NHS about 7.00 £ per month as of 2019.[2] In the United States, the wholesale cost of this amount is about 2.15 USD.[6] In 2016 it was the 61st most prescribed medication in the United States with more than 12 million prescriptions.[7]
Medical uses
Glimepiride is indicated to treat type 2 diabetes mellitus; its mode of action is to increase insulin secretion by the pancreas. However it requires adequate insulin synthesis as prerequisite to treat appropriately. It is not used for type 1 diabetes because in type 1 diabetes the pancreas is not able to produce insulin.[8]
Contraindications
Its use is contraindicated in patients with hypersensitivity to glimepiride or other sulfonylureas.
Adverse effects
Side effects from taking glimepiride include gastrointestinal tract (GI) disturbances, occasional allergic reactions, and rarely blood production disorders including thrombocytopenia, leukopenia, and hemolytic anemia. In the initial weeks of treatment, the risk of hypoglycemia may be increased. Alcohol consumption and exposure to sunlight should be restricted because they can worsen side effects.[8]
Interactions
Nonsteroidal anti-inflammatory drugs (such as salicylates), sulfonamides, chloramphenicol, coumadin and probenecid may potentiate the hypoglycemic action of glimepiride. Thiazides, other diuretics, phothiazides, thyroid products, oral contraceptives, and phenytoin tend to produce hyperglycemia.
Mechanism of action
Like all sulfonylureas, glimepiride acts as an insulin secretagogue.[9] It lowers blood sugar by stimulating the release of insulin by pancreatic beta cells and by inducing increased activity of intracellular insulin receptors.
Not all secondary sulfonylureas have the same risk of hypoglycemia. Glibenclamide (glyburide) is associated with an incidence of hypoglycemia of up to 20–30%, compared to as low as 2% to 4% with glimepiride. Glibenclamide also interferes with the normal homeostatic suppression of insulin secretion in reaction to hypoglycemia, whereas glimepiride does not. Also, glibenclamide diminishes glucagon secretion in reaction to hypoglycemia, whereas glimepiride does not.[10]
Pharmacokinetics
Gastrointestinal absorption is complete, with no interference from meals. Significant absorption can occur within one hour, and distribution is throughout the body, 99.5% bound to plasma protein. Metabolism is by oxidative biotransformation, it is hepatic and complete. First, the medication is metabolized to M1 metabolite by CYP2C9. M1 possesses about 1⁄3 of pharmacological activity of glimepiride, yet it is unknown if this results in clinically meaningful effect on blood glucose. M1 is further metabolized to M2 metabolite by cytosolic enzymes. M2 is pharmacologically inactive. Excretion in the urine is about 65%, and the remainder is excreted in the feces.
References
- "Glimepiride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 693. ISBN 9780857113382.
- "Glimepiride Pregnancy and Breastfeeding Warnings". Drugs.com. Retrieved 3 March 2019.
- Davis SN (2004). "The role of glimepiride in the effective management of Type 2 diabetes". J. Diabetes Complicat. 18 (6): 367–76. doi:10.1016/j.jdiacomp.2004.07.001. PMID 15531188.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 449. ISBN 9783527607495.
- "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Retrieved 3 March 2019.
- "The Top 300 of 2019". clincalc.com. Retrieved 22 December 2018.
- "Glimepiride: MedlinePlus Drug Information". nih.gov.
- Nissen SE, Nicholls SJ, Wolski K, et al. (April 2008). "Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial". JAMA. 299 (13): 1561–73. doi:10.1001/jama.299.13.1561. PMID 18378631.
- Davis, Stephen N. (2005). "60. Insulin, oral hypoglycemic agents, and the pharmacology of the endocrine pancreas". In Brunton, Laurence L.; Lazo, John S.; Parker, Keith L. (eds.) (eds.). Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw-Hill. p. 1636. ISBN 0-07-142280-3.CS1 maint: uses editors parameter (link)