Muraglitazar
Muraglitazar (proposed tradename Pargluva) is a dual peroxisome proliferator-activated receptor agonist with affinity to PPARα and PPARγ.[1]
 | |
| Clinical data | |
|---|---|
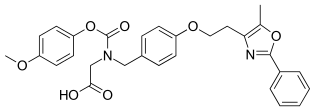
| Other names | 2-[(4-Methoxyphenoxy)carbonyl-[[4-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]phenyl]methyl]amino]acetic acid |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C29H28N2O7 |
| Molar mass | 516.54 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
The drug had completed phase III clinical trials,[2] however in May, 2006 Bristol-Myers Squibb announced that it had discontinued further development.[3]
Data on muraglitazar is relatively sparse due to the recent introduction of this agent. One double-blind randomized clinical trial[2] comparing muraglitazar and pioglitazone found that the effects of the former were favourable in terms of HDL-C increase, decrease in total cholesterol, apolipoprotein B, triglycerides and a greater reduction in HbA1c (p <0.0001 for all comparisons). However, the muraglitazar group had a higher all-cause mortality, greater incidence of edema and heart failure and more weight gain compared to the pioglitazone group. A meta-analysis of the phase II and III clinical trials of muraglitazar revealed that it was associated with a greater incidence of myocardial infarction, stroke, transient ischemic attacks and congestive heart failure (CHF) when compared to placebo or pioglitazone.[4]
References
- Waites, CR; Dominick, MA; Sanderson, TP; Schilling, BE (13 August 2007). "Nonclinical Safety Evaluation of Muraglitazar, a Novel PPARα/γ Agonist" (PDF). Toxicological Sciences. 100 (1): 248–58. doi:10.1093/toxsci/kfm193. PMID 17675651. Retrieved 9 November 2016.
- Kendall, DM; Rubin, CJ; Mohideen, P; Ledeine, JM; Belder, R; Gross, J; Norwood, P; O'Mahony, M; Sall, K; Sloan, G; Roberts, A; Fiedorek, FT; DeFronzo, RA (May 2006). "Improvement of Glycemic Control, Triglycerides, and HDL Cholesterol Levels with Muraglitazar, a Dual (α/γ) Peroxisome Proliferator–Activated Receptor Activator, in Patients with Type 2 Diabetes Inadequately Controlled with Metformin Monotherapy" (PDF). Diabetes Care. 29 (5): 1016–23. doi:10.2337/diacare.2951016. PMID 16644631. Retrieved 9 November 2016.
- "Bristol-Myers Squibb Announces Discontinuation of Development of Muraglitazar, an Investigational Oral Treatment for Type 2 Diabetes". PR Newswire from Bristol-Myers Squibb. May 18, 2006. Retrieved 9 November 2016.
- Nissen, SE; Wolski, K; Topol, EJ (23 November 2005). "Effect of Muraglitazar on Death and Major Adverse Cardiovascular Events in Patients with Type 2 Diabetes Mellitus". Journal of the American Medical Association. 294 (20): 2581–6. doi:10.1001/jama.294.20.joc50147. PMID 16239637.