N-type calcium channel
N-type calcium channels are voltage gated calcium channels that are distributed throughout the entire body. These channels are high voltage activated channels composed of alpha-1B subunits.[1] The alpha subunit forms the pore through which the calcium enters and helps to determine most of the channel's properties. The alpha subunit is also known as the calcium channel/voltage dependent/N type, alpha 1 subunit (CACNA1B), or calcium voltage-gated channel subunit alpha1 B. The subunit is essential to modulate neurotransmitter release. They also contain associated subunits such as β1, β3, β4, α2δ, and possibly γ.[2] These channels are known for their importance in the nervous system. They play a small role in the migration of immature neurons before the establishment of their mature synapses, and they are critically involved in the release of neurotransmitters, which is also similar to another type of calcium channels, known as P-type calcium channels.[2] N-type calcium channels are targets for the development of drugs to relieve chronic and neuropathic pain. They are also used for the treatment of hypertension, autism spectrum disorder, osteoarthritis, and other medical diagnoses. Additionally, N-type calcium channels have known functions in the kidney, and heart. There are many known N-type calcium channel blockers that function to inhibit channel activity, although the most notable blockers are ω-Conotoxins. Blockers, like ω-Conotoxins, can interfere with many different biological and therapeutic processes.[3]
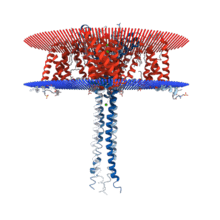
Structure
N-type calcium channels are categorized as high threshold-activated channels and seen in the Cav2 gene family. The structure of the N-type calcium channel is very similar to other voltage-dependent channels. Alpha, beta, and gamma subunits show that those subunits are substrates for cAMP-dependent protein phosphorylation. The most important part of the channel is the actual pore that is formed by the alpha-1 subunit. This pore is the location of the import of the extracellular ions. The alpha 1 subunit has as many as 2000 amino acid residues within an amino acid sequence with the transmembrane structure with a pore. This is organized into 6 six segments(S1-S6). S1, S2, S3, S5, and S6 are hydrophobic while S4 serves as the voltage-sensor. In addition there is a membrane-associated loop in between S5 and S6. The activity of the pore is modulated by 4 subunits: an intracellular β-subunit, a transmembrane gamma subunit, and complex of alpha-2 and delta subunits.[4]
In addition to the α1 subunit, the following subunits are present in the N-type calcium channel:
- α2δ – CACNA2D1, CACNA2D2
- β1 – CACNB1
- β3 – CACNB3
- β4 – CACNB4
Function
N-type calcium channels are highly known for their function in the nervous system, but they are also involved with the function of the heart and kidneys.[5] They are important in neurotransmitter release because they are localized at the synaptic terminals.[6] In the peripheral nervous system, N-type channels are known to be involved in the release of many neurotransmitters like glutamate, GABA, acetylcholine, dopamine, and norepinephrine. When extracellular calcium flows into N-type calcium channels due to an action potential, it triggers the fusion of the secretory vesicles. Studies on the cardiovascular system reveal when ω-Conotoxin is introduced, it causes the inhibition of norepinephrine, and this shows that only the N-type calcium channel, not the P/Q/L type calcium channels, are involved in the release of norepinephrine.[7] In the Kidneys, blocking of N-type calcium channels reduce glomerular pressure through dilation of arteries.[5] The inhibition of this channel by calcium channel blockers can lead to renal microcirculation. N-type calcium channels have been shown to play a part in the localization of neurite growth in the sympathetic nervous system and the skin and spinal cord. The neurite outgrowth was shown to be inhibited through an interaction between laminin and the 11th loop of the n-type calcium channel structure.[8] It has been suggested that neuritis outgrowth is inhibited by the influx of calcium through the growth cone, and this happens when the Cav2.2 subunit comes in contact with laminin 2, and in response can induce a stretch activation of the N-type calcium channel.[8]
Blockers
N-type calcium channel blockers play a large role in pain regulation. In the pain pathway, N-type Calcium channels serve to regulate pain signals sent from the peripheral nervous system to Central Nervous System. There are many known blockers that function very similarly to one another, but the most common are the conotoxins. To block a voltage-gated channel, the voltage current that activates the channels needs to be stopped. These toxins and blockers generally function by modulating G-Protein Coupled Receptors (GPCR) that mediate the N-type Calcium channels. This modulation of GPCRs activates GABA, an inhibitory neurotransmitter. This GABA results in the inhibition of pain signals.[9]
List of N-type Calcium channel blockers:
- ω-Conotoxins
- Cadmium
- Caroverine
- Cilnidipine
- Gabapentin
- Levetiracetam
- Lamotrigine
- Nicardipine
- NP078585
- Pregabalin
- TROX-1
- Ziconotide
There are many more blockers being researched.
Mutation studies
.jpg)
When the N-type calcium channels are mutated, it can lead to problems in its function and can also lead to clinical problems. Most mutations occur within the Alpha subunit of the channel, the CACNA1B gene. Mutations of said gene have been known to directly correlate with several neuropsychiatric diseases. For example, a missense mutation can lead to Myoclonus-Dystonia syndrome, where as a duplication can lead to Autism. Mutation in the N-type channel can also cause bipolar disorder and schizophrenia, two heritable diseases with overlapping genetic components. Studies have also shown that deletion of the α2δ subunit can alleviate neuropathic inflammatory pain, specifically.[10]
Clinical significance
N-type calcium channels have been connected to a variety of different clinical diagnoses. The alteration of N-type calcium channels in therapeutic processes occurs in four major ways; through the blockage of N-type calcium channel peptides, interference of the flow of ions through the channel itself, activation of G-protein coupled signaling, and interference of the G-protein pathways.[11] They are most commonly linked to therapeutic treatment of chronic pain. Studies have shown that the intrathecal injection of calcium channel inhibitors such as Ziconotide, to block the N-type calcium channels, have produced alleviation of intractable pain.[12] Administering N-type peptide blockers via injection into the spinal canal (intrathecal injection) allows the drugs to reach the cerebral spinal fluid where the channels are located. The use of blockers to inhibit the N-type calcium channels have produced alleviation of chronic pain from a variety of different diseases. For example, blockade of the N-type calcium channel is a potential therapeutic strategy for the treatment of alcoholism using N-type channel antagonists. Because prolonged alcohol exposure over time has been known to increase N-type channel function, experiments have been performed to determine the effect inhibiting them has on alcohol addiction. Studies have shown that using N-type antagonists to decrease channel activity has shown reduced voluntary consumption of alcohol.[13]
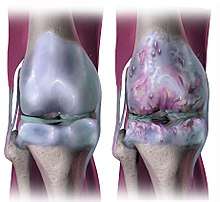
Studies have also shown that N-type peptide blockers have been used to relieve pain that results from osteoarthritis, hypertension, diabetic neuropathy. It has also been known to be used to aid in relief of pain associated with cancer and can be used for patients actively battling cancer. Studies are being done to see if the treatment may also be used for those survivors still experiencing chronic pain. Also, due to the N-type calcium channel being intrinsic to neurotransmitter release and excitability, they can be regulated via GPCR signaling using Analgesic opioid drugs to relieve pain. This is done by modulating the sensory transmitter release and subsequently lessening the feelings of pain [14] and cancer. However, the use of intrathecally injected N-type channel blockers has proven to be more beneficial than common opioid remedies because there are less negative side effects associated.[15]
The Cav2.2 channel inhibitor, Ziconotide, for example, is the only drug on the market as of now that directly targets the n-type channel and its conotoxin peptide. N-type calcium channels have also been associated with several known diseases as well. For example, mutations in the CACNA1B gene have been associated with Myoclonus-Dystonia syndrome, an unusual hyperkinetic movement disorder with muscular contractile symptoms. This can be explained by the fact that the channel is mutated with an increase in current flow, subsequently causing hyperexcitability. It has also been linked to cardiac arrhythmia, as increased excitability can cause tachycardia. Issues with the alpha subunit of the N-type calcium channel .[10] Duplication of the CACNA1B gene has also been linked to cases of the autism spectrum disorder as well as psychiatric diseases such as bipolar disorder and schizophrenia.
.[16]
N-Type channel and pain regulation
N-type calcium voltage gated ion channels are permeable to calcium and are known to regulate neuronal excitability and the firing of action potentials in the neurons. These actions affect the transmission of neurotransmitters in nociceptive pathways. Nociceptive pathways are the neurons that are involved in pain perception, which typically arises from damage of the neurons or disease occurring in the nerves themselves. When action potentials in this pathway make it to the central terminals of the sensory neurons that are in the spinal cord, there is an influx of calcium that enters through the voltage-gated ion channels. This influx of calcium then triggers the release of the pro-nociceptive neurotransmitters. These neurotransmitters then bind to the receptors on the sensory neurons that cause a person to feel pain. Since these channels are able to influence the neurotransmitters in the body that cause a person to sense pain, voltage-gated ion channels that are permeable to calcium have also been found to be good targets for regulating chronic pain in patients who have difficulty finding effective treatment. A few of the conditions involving pain that are difficult to treat are acute pain, chronic inflammatory pain, and chronic neuropathic pain. These conditions are treated with medicine that has either been prone to becoming addictive to the patient, or can cause serious side effects, making the medicine controversial.[17]
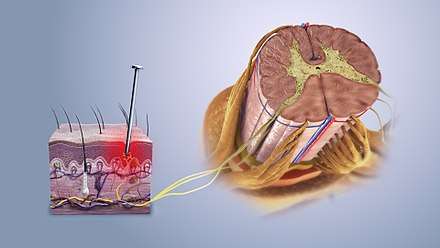
Studies have shown that N-type channels are particularly of interest in developing new medicine to help in treating chronic pain. N-type channels play a key role in being able to control the neurotransmission of pain in the spinal cord. Studies have shown that N-type channels are located in high amounts at the presynaptic terminals of neurons. They have a complex of proteins that form a pore with the ɑ1Bsubunit (also known as CaV2.2) and an auxiliary ɑ2δδ and β subunits. When there is tissue damage, these subunits are regulated in the neuron, which helps to prove that these channels are involved in nociceptive transmission. When looking at the spinal cord, the distribution of N-type channels also coincides with the evidence of these channels having a role in the transmission of nociceptive information in the body. A new drug, Ziconotide, is being tested to alter N-type channels in order to treat these conditions of chronic pain. One of the newest medicines is an analgesic drug that works through targeting N-type calcium channels specifically in order to lessen the transmission of pain. Peptide ɷɷ-conotoxins are what bind to the N-type calcium channels in order to block ion permeation, which then blocks the influx of calcium and the transmission of the nociceptive responses. Neurons located in the posterior horn of the spinal cord experience ɷɷ-conotoxins that, when binding to the receptors, can inhibit the release of the pro-nociceptive neurotransmitters and neuromodulators that come from the central nerve terminals of the afferent neurons. This inhibition is what ultimately can stop the transmission of pain to the person from damaged cells. Ziconotide is an inorganic version of the ɷɷ-conotoxin, MVIIA. This medicine is able to inhibit the release of pro-nociceptive receptors, which will then block the transmission of pain.[17]
References
- Williams ME, Brust PF, Feldman DH, Patthi S, Simerson S, Maroufi A, McCue AF, Veliçelebi G, Ellis SB, Harpold MM (July 1992). "Structure and functional expression of an omega-conotoxin-sensitive human N-type calcium channel". Science. 257 (5068): 389–95. Bibcode:1992Sci...257..389W. doi:10.1126/science.1321501. PMID 1321501.
- "Voltage-dependent calcium channel, N-type, alpha-1 subunit". InterPro. EMBL-EBI.
- Adams DJ, Berecki G (July 2013). "Mechanisms of conotoxin inhibition of N-type (Ca(v)2.2) calcium channels". Biochimica et Biophysica Acta. 1828 (7): 1619–28. doi:10.1016/j.bbamem.2013.01.019. PMID 23380425.
- EMBL-EBI, InterPro. "Voltage-dependent calcium channel, N-type, alpha-1 subunit (IPR005447) < InterPro < EMBL-EBI". www.ebi.ac.uk.
- Hayashi K, Wakino S, Sugano N, Ozawa Y, Homma K, Saruta T (February 2007). "Ca2+ channel subtypes and pharmacology in the kidney". Circulation Research. 100 (3): 342–53. doi:10.1161/01.RES.0000256155.31133.49. PMID 17307972.
- Weber AM, Wong FK, Tufford AR, Schlichter LC, Matveev V, Stanley EF (2010). "N-type Ca2+ channels carry the largest current: implications for nanodomains and transmitter release". Nature Neuroscience. 13 (11): 1348–50. doi:10.1038/nn.2657. PMID 20953196. Lay summary – NeuroScience: Plus Biology.
- Molderings GJ, Likungu J, Göthert M (February 2000). "N-Type calcium channels control sympathetic neurotransmission in human heart atrium". Circulation. 101 (4): 403–7. doi:10.1161/01.cir.101.4.403. PMID 10653832.
- Weiss N (May 2008). "The N-type voltage-gated calcium channel: when a neuron reads a map". The Journal of Neuroscience. 28 (22): 5621–2. doi:10.1523/JNEUROSCI.1538-08.2008. PMID 18509022.
- Adams, David J.; Berecki, Géza (2013-07-01). "Mechanisms of conotoxin inhibition of N-type (Cav2.2) calcium channels". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1828 (7): 1619–1628. doi:10.1016/j.bbamem.2013.01.019. ISSN 0005-2736. PMID 23380425.
- Groen, Justus L.; Andrade, Arturo; Ritz, Katja; Jalalzadeh, Hamid; Haagmans, Martin; Bradley, Ted E.J.; Jongejan, Aldo; Verbeek, Dineke S.; Nürnberg, Peter; Denome, Sylvia; Hennekam, Raoul C.M.; Lipscombe, Diane; Baas, Frank; Tijssen, Marina A.J. (15 February 2015). "CACNA1B mutation is linked to unique myoclonus-dystoniasyndrome". Human Molecular Genetics. 24 (4): 987–993. doi:10.1093/hmg/ddu513. PMC 4817404. PMID 25296916.
- Zamponi GW, Striessnig J, Koschak A, Dolphin AC (October 2015). "The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential". Pharmacological Reviews. 67 (4): 821–70. doi:10.1124/pr.114.009654. PMC 4630564. PMID 26362469.
- Dray A, Read SJ (May 2007). "Arthritis and pain. Future targets to control osteoarthritis pain". Arthritis Research & Therapy. 9 (3): 212. doi:10.1186/ar2178. PMC 2206352. PMID 17561993.
- Newton PM, Zeng L, Wang V, Connolly J, Wallace MJ, Kim C, Shin HS, Belardetti F, Snutch TP, Messing RO (November 2008). "A blocker of N- and T-type voltage-gated calcium channels attenuates ethanol-induced intoxication, place preference, self-administration, and reinstatement". The Journal of Neuroscience. 28 (45): 11712–9. doi:10.1523/JNEUROSCI.3621-08.2008. PMC 3045811. PMID 18987207.
- Javed S, Petropoulos IN, Alam U, Malik RA (January 2015). "Treatment of painful diabetic neuropathy". Therapeutic Advances in Chronic Disease. 6 (1): 15–28. doi:10.1177/2040622314552071. PMC 4269610. PMID 25553239.
- Bruel BM, Burton AW (December 2016). "Intrathecal Therapy for Cancer-Related Pain". Pain Medicine. 17 (12): 2404–2421. doi:10.1093/pm/pnw060. PMC 5654346. PMID 28025375.
- Heyes S, Pratt WS, Rees E, Dahimene S, Ferron L, Owen MJ, Dolphin AC (November 2015). "Genetic disruption of voltage-gated calcium channels in psychiatric and neurological disorders". Progress in Neurobiology. 134: 36–54. doi:10.1016/j.pneurobio.2015.09.002. PMC 4658333. PMID 26386135.
- McGivern, Joseph G. (1 March 2006). "Targeting N-type and T-type calcium channels for the treatment of pain". Drug Discovery Today. 11 (5–6): 245–253. doi:10.1016/S1359-6446(05)03662-7. PMID 16580601.
Further reading
- Calabrese B, Tabarean IV, Juranka P, Morris CE (November 2002). "Mechanosensitivity of N-type calcium channel currents". Biophysical Journal. 83 (5): 2560–74. Bibcode:2002BpJ....83.2560C. doi:10.1016/S0006-3495(02)75267-3. PMC 1302342. PMID 12414690.
- Moskvina V, Craddock N, Holmans P, Nikolov I, Pahwa JS, Green E, Owen MJ, O'Donovan MC (March 2009). "Gene-wide analyses of genome-wide association data sets: evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk". Molecular Psychiatry. 14 (3): 252–60. doi:10.1038/mp.2008.133. PMC 3970088. PMID 19065143.
- Castiglioni AJ, Raingo J, Lipscombe D (October 2006). "Alternative splicing in the C-terminus of CaV2.2 controls expression and gating of N-type calcium channels". The Journal of Physiology. 576 (Pt 1): 119–34. doi:10.1113/jphysiol.2006.115030. PMC 1995641. PMID 16857708.
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J (December 2005). "International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels". Pharmacological Reviews. 57 (4): 411–25. doi:10.1124/pr.57.4.5. PMID 16382099.
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (November 2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell. 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983.
- Stotz SC, Barr W, McRory JE, Chen L, Jarvis SE, Zamponi GW (January 2004). "Several structural domains contribute to the regulation of N-type calcium channel inactivation by the beta 3 subunit". The Journal of Biological Chemistry. 279 (5): 3793–800. doi:10.1074/jbc.M308991200. PMID 14602720.
- Maximov A, Bezprozvanny I (August 2002). "Synaptic targeting of N-type calcium channels in hippocampal neurons" (PDF). The Journal of Neuroscience. 22 (16): 6939–52. doi:10.1523/JNEUROSCI.22-16-06939.2002. PMC 3307533. PMID 12177192.
- Peng S, Hajela RK, Atchison WD (December 2002). "Characteristics of block by Pb2+ of function of human neuronal L-, N-, and R-type Ca2+ channels transiently expressed in human embryonic kidney 293 cells". Molecular Pharmacology. 62 (6): 1418–30. doi:10.1124/mol.62.6.1418. PMID 12435810.
- Murakami M, Fleischmann B, De Felipe C, Freichel M, Trost C, Ludwig A, Wissenbach U, Schwegler H, Hofmann F, Hescheler J, Flockerzi V, Cavalié A (October 2002). "Pain perception in mice lacking the beta3 subunit of voltage-activated calcium channels". The Journal of Biological Chemistry. 277 (43): 40342–51. doi:10.1074/jbc.M203425200. PMID 12161429.
- Vitko I, Shcheglovitov A, Baumgart JP, Arias-Olguín II, Murbartián J, Arias JM, Perez-Reyes E (2008). Schwartz A (ed.). "Orientation of the calcium channel beta relative to the alpha(1)2.2 subunit is critical for its regulation of channel activity". PLOS ONE. 3 (10): e3560. Bibcode:2008PLoSO...3.3560V. doi:10.1371/journal.pone.0003560. PMC 2570331. PMID 18958281.
- Agler HL, Evans J, Tay LH, Anderson MJ, Colecraft HM, Yue DT (June 2005). "G protein-gated inhibitory module of N-type (ca(v)2.2) ca2+ channels". Neuron. 46 (6): 891–904. doi:10.1016/j.neuron.2005.05.011. PMID 15953418.