Voltage-gated ion channel
Voltage-gated ion channels are a class of transmembrane proteins that form ion channels that are activated by changes in the electrical membrane potential near the channel. The membrane potential alters the conformation of the channel proteins, regulating their opening and closing. Cell membranes are generally impermeable to ions, thus they must diffuse through the membrane through transmembrane protein channels. They have a crucial role in excitable cells such as neuronal and muscle tissues, allowing a rapid and co-ordinated depolarization in response to triggering voltage change. Found along the axon and at the synapse, voltage-gated ion channels directionally propagate electrical signals. Voltage-gated ion-channels are usually ion-specific, and channels specific to sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) ions have been identified.[1] The opening and closing of the channels are triggered by changing ion concentration, and hence charge gradient, between the sides of the cell membrane.[2]
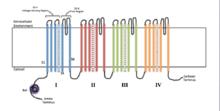 Each of the four homologous domains makes up one subunit of the ion channel. The S4 voltage-sensing segments (marked with + symbols) are shown as charged. | |
| Identifiers | |
|---|---|
| Symbol | VIC |
| Pfam clan | CL0030 |
| TCDB | 1.A.1 |
| OPM superfamily | 8 |
| OPM protein | 2a79 |
Structure
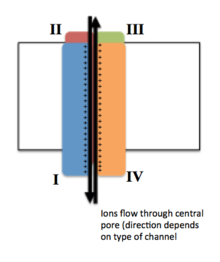
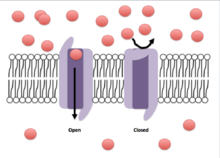
Voltage-gated ion channels are generally composed of several subunits arranged in such a way that there is a central pore through which ions can travel down their electrochemical gradients. The channels tend to be ion-specific, although similarly sized and charged ions may sometimes travel through them. The functionality of voltage-gated ion channels is attributed to its three main discrete units: the voltage sensor, the pore or conducting pathway, and the gate.[3] Na+, K+, and Ca2+ channels are composed of four transmembrane domains arranged around a central pore; these four domains are part of a single α-subunit in the case of most Na+ and Ca2+ channels, whereas there are four α-subunits, each contributing one transmembrane domain, in most K+ channels.[4] The membrane-spanning segments, designated S1-S6, all take the form of alpha helices with specialized functions. The fifth and sixth transmembrane segments (S5 and S6) and pore loop serve the principal role of ion conduction, comprising the gate and pore of the channel, while S1-S4 serve as the voltage-sensing region.[3] The four subunits may be identical, or different from one another. In addition to the four central α-subunits, there are also regulatory β-subunits, with oxidoreductase activity, which are located on the inner surface of the cell membrane and do not cross the membrane, and which are coassembled with the α-subunits in the endoplasmic reticulum.[5]
Mechanism
Crystallographic structural studies of a potassium channel have shown that, when a potential difference is introduced over the membrane, the associated electric field induces a conformational change in the potassium channel. The conformational change distorts the shape of the channel proteins sufficiently such that the cavity, or channel, opens to allow influx or efflux to occur across the membrane. This movement of ions down their concentration gradients subsequently generates an electric current sufficient to depolarize the cell membrane.
Voltage-gated sodium channels and calcium channels are made up of a single polypeptide with four homologous domains. Each domain contains 6 membrane spanning alpha helices. One of these helices, S4, is the voltage sensing helix.[6] The S4 segment contains many positive charges such that a high positive charge outside the cell repels the helix, keeping the channel in its closed state.
In general, the voltage sensing portion of the ion channel is responsible for the detection of changes in transmembrane potential that trigger the opening or closing of the channel. The S1-4 alpha helices are generally thought to serve this role. In potassium and sodium channels, voltage-sensing S4 helices contain positively-charged lysine or arginine residues in repeated motifs.[3] In its resting state, half of each S4 helix is in contact with the cell cytosol. Upon depolarization, the positively-charged residues on the S4 domains move toward the exoplasmic surface of the membrane. It is thought that the first 4 arginines account for the gating current, moving toward the extracellular solvent upon channel activation in response to membrane depolarization. The movement of 10–12 of these protein-bound positive charges triggers a conformational change that opens the channel.[4] The exact mechanism by which this movement occurs is not currently agreed upon, however the canonical, transporter, paddle, and twisted models are examples of current theories.[7]
Movement of the voltage-sensor triggers a conformational change of the gate of the conducting pathway, controlling the flow of ions through the channel.[3]
The main functional part of the voltage-sensitive protein domain of these channels generally contains a region composed of S3b and S4 helices, known as the "paddle" due to its shape, which appears to be a conserved sequence, interchangeable across a wide variety of cells and species. A similar voltage sensor paddle has also been found in a family of voltage sensitive phosphatases in various species.[8] Genetic engineering of the paddle region from a species of volcano-dwelling archaebacteria into rat brain potassium channels results in a fully functional ion channel, as long as the whole intact paddle is replaced.[9] This "modularity" allows use of simple and inexpensive model systems to study the function of this region, its role in disease, and pharmaceutical control of its behavior rather than being limited to poorly characterized, expensive, and/or difficult to study preparations.[10]
Although voltage-gated ion channels are typically activated by membrane depolarization, some channels, such as inward-rectifier potassium ion channels, are activated instead by hyperpolarization.
The gate is thought to be coupled to the voltage sensing regions of the channels and appears to contain a mechanical obstruction to ion flow.[11] While the S6 domain has been agreed upon as the segment acting as this obstruction, its exact mechanism is unknown. Possible explanations include: the S6 segment makes a scissor-like movement allowing ions to flow through,[12] the S6 segment breaks into two segments allowing of passing of ions through the channel,[13] or the S6 channel serving as the gate itself.[14] The mechanism by which the movement of the S4 segment affects that of S6 is still unknown, however it is theorized that there is a S4-S5 linker whose movement allows the opening of S6.[3]
Inactivation of ion channels occurs within milliseconds after opening. Inactivation is thought to be mediated by an intracellular gate that controls the opening of the pore on the inside of the cell.[15] This gate is modeled as a ball tethered to a flexible chain. During inactivation, the chain folds in on itself and the ball blocks the flow of ions through the channel.[16] Fast inactivation is directly linked to the activation caused by intramembrane movements of the S4 segments,[17] though the mechanism linking movement of S4 and the engagement of the inactivation gate is unknown.
Different types
Sodium (Na+) channels
Sodium channels have similar functional properties across many different cell types. While ten human genes encoding for sodium channels have been identified, their function is typically conserved between species and different cell types.[17]
Calcium (Ca2+) channels
With sixteen different identified genes for human calcium channels, this type of channel differs in function between cell types. Ca2+ channels produce action potentials similarly to Na+ channels in some neurons. They also play a role in neurotransmitter release in pre-synaptic nerve endings. In most cells, Ca2+ channels regulate a wide variety of biochemical processes due to their role in controlling intracellular Ca2+ concentrations.[13]
Potassium (K+) channels
Potassium channels are the largest and most diverse class of voltage-gated channels, with over 100 encoding human genes. These types of channels differ significantly in their gating properties; some inactivating extremely slowly and others inactivating extremely quickly. This difference in activation time influences the duration and rate of action potential firing, which has a significant effect on electrical conduction along an axon as well as synaptic transmission. Potassium channels differ in structure from the other channels in that they contain four separate polypeptide subunits, while the other channels contain four homologous domain but on a single polypeptide unit.[7]
Chloride (Cl−) channels
Chloride channels are present in all types of neurons. With the chief responsibility of controlling excitability, chloride channels contribute to the maintenance of cell resting potential and help to regulate cell volume.[1]
Phylogenetics
Phylogenetic studies of proteins expressed in bacteria revealed the existence of a superfamily of voltage-gated sodium channels.[21] Subsequent studies have shown that a variety of other ion channels and transporters are phylogenetically related to the voltage-gated ion channels, including inwardly rectifying K+ channels, ryanodine-inositol 1,4,5-triphosphate receptor Ca2+ channels, transient receptor potential Ca2+ channels, polycystin cation channels, glutamate-gated ion channels, calcium-dependent chloride channels, monovalent cation:proton antiporters, type 1, and potassium transporters.[22]
References
- Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A, McNamara JO, Williams SM (2001). "Voltage-Gated Ion Channels". Neuroscience (2nd ed.). Sunderland, Mass: Sinauer Associates. ISBN 978-0-87893-742-4.
- Catterall WA (April 2000). "From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels". Neuron. 26 (1): 13–25. doi:10.1016/S0896-6273(00)81133-2. PMID 10798388.
- Bezanilla F (March 2005). "Voltage-gated ion channels". IEEE Transactions on NanoBioscience. 4 (1): 34–48. doi:10.1109/tnb.2004.842463. PMID 15816170.
- Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (2000). "Section 21.3, Molecular Properties of Voltage-Gated Ion Channels". Molecular Cell Biology (4th ed.). New York: Scientific American Books. ISBN 978-0-7167-3136-8.
- Gulbis JM, Mann S, MacKinnon R (June 1999). "Structure of a voltage-dependent K+ channel beta subunit". Cell. 97 (7): 943–52. doi:10.1016/s0092-8674(00)80805-3. PMID 10399921.
- Catterall WA (2010). "Ion channel voltage sensors: structure, function, and pathophysiology". Neuron. 67 (6): 915–28. doi:10.1016/j.neuron.2010.08.021. PMC 2950829. PMID 20869590.
- Sands Z, Grottesi A, Sansom MS (2005). "Voltage-gated ion channels". Current Biology. 15 (2): R44–7. doi:10.1016/j.cub.2004.12.050. PMID 15668152.
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y (June 2005). "Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor". Nature. 435 (7046): 1239–43. Bibcode:2005Natur.435.1239M. doi:10.1038/nature03650. PMID 15902207.
- Alabi AA, Bahamonde MI, Jung HJ, Kim JI, Swartz KJ (November 2007). "Portability of paddle motif function and pharmacology in voltage sensors". Nature. 450 (7168): 370–5. Bibcode:2007Natur.450..370A. doi:10.1038/nature06266. PMC 2709416. PMID 18004375.
- Long SB, Tao X, Campbell EB, MacKinnon R (November 2007). "Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment". Nature. 450 (7168): 376–82. Bibcode:2007Natur.450..376L. doi:10.1038/nature06265. PMID 18004376.
- Yellen G (August 1998). "The moving parts of voltage-gated ion channels". Quarterly Reviews of Biophysics. 31 (3): 239–95. doi:10.1017/s0033583598003448. PMID 10384687.
- Perozo E, Cortes DM, Cuello LG (July 1999). "Structural rearrangements underlying K+-channel activation gating". Science. 285 (5424): 73–8. doi:10.1126/science.285.5424.73. PMID 10390363.
- Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R (May 2002). "Crystal structure and mechanism of a calcium-gated potassium channel". Nature. 417 (6888): 515–22. Bibcode:2002Natur.417..515J. doi:10.1038/417515a. PMID 12037559.
- Webster SM, Del Camino D, Dekker JP, Yellen G (April 2004). "Intracellular gate opening in Shaker K+ channels defined by high-affinity metal bridges". Nature. 428 (6985): 864–8. Bibcode:2004Natur.428..864W. doi:10.1038/nature02468. PMID 15103379.
- Armstrong CM (July 1981). "Sodium channels and gating currents". Physiological Reviews. 61 (3): 644–83. doi:10.1152/physrev.1981.61.3.644. PMID 6265962.
- Vassilev P, Scheuer T, Catterall WA (October 1989). "Inhibition of inactivation of single sodium channels by a site-directed antibody". Proceedings of the National Academy of Sciences of the United States of America. 86 (20): 8147–51. Bibcode:1989PNAS...86.8147V. doi:10.1073/pnas.86.20.8147. PMC 298232. PMID 2554301.
- Bénitah JP, Chen Z, Balser JR, Tomaselli GF, Marbán E (March 1999). "Molecular dynamics of the sodium channel pore vary with gating: interactions between P-segment motions and inactivation". The Journal of Neuroscience. 19 (5): 1577–85. doi:10.1523/JNEUROSCI.19-05-01577.1999. PMC 6782169. PMID 10024345.
- Cherny, V.V.; Markin, V.S.; DeCoursey, T.E. (1995), "The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient", Journal of General Physiology (published June 1995), 105 (6), pp. 861–896, doi:10.1085/jgp.105.6.861, PMC 2216954, PMID 7561747
- DeCoursey, T.E. (2003), "Voltage-gated proton channels and other proton transfer pathways", Physiological Reviews, 83 (2), pp. 475–579, doi:10.1152/physrev.00028.2002, OCLC 205658168, PMID 12663866
- Ramsey, I. Scott; Mokrab, Younes; Carvacho, Ingrid; Sands, Zara A.; Sansom, Mark S.P.; Clapham, David E. (2010). "An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1". Nature Structural & Molecular Biology. 17 (7): 869–875. doi:10.1038/nsmb.1826. PMC 4035905. PMID 20543828.
- Koishi R, Xu H, Ren D, Navarro B, Spiller BW, Shi Q, Clapham DE (March 2004). "A superfamily of voltage-gated sodium channels in bacteria". The Journal of Biological Chemistry. 279 (10): 9532–8. doi:10.1074/jbc.M313100200. PMID 14665618.
- Chang, Abraham B.; Lin, Ron; Studley, W. Keith; Tran, Can V.; Saier, Milton H., Jr. (2004). "Phylogeny as a guide to structure and function of membrane transport proteins". Mol Membr Biol. 21 (3): 171–181. doi:10.1080/09687680410001720830. PMID 15204625.
External links
- IUPHAR-DB Voltage-gated ion channel subunits
- The IUPHAR Compendium of Voltage-gated Ion Channels 2005
- Voltage-Dependent+Anion+Channels at the US National Library of Medicine Medical Subject Headings (MeSH)