Mesuximide
Mesuximide (or methsuximide, methosuximide) is a succinimide anticonvulsant medication. It is sold as a racemate by Pfizer under the tradenames Petinutin (Switzerland)[1] and Celontin (United States).[2] The therapeutic efficacy of methosuximide is largely due to its pharmacologically active metabolite, N-desmethylmethosuximide, which has a longer half-life and attains much higher plasma levels than its parent.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Celontin |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a682028 |
| Pregnancy category |
|
| Routes of administration | By mouth (capsules) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic (demethylation and glucuronidation) |
| Metabolites | N-desmethylmethosuximide |
| Elimination half-life | 1.4–2.6 hours (mesuximide) 28–38 hours (active metabolite) |
| Excretion | Urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.934 |
| Chemical and physical data | |
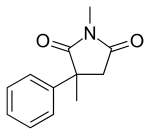
| Formula | C12H13NO2 |
| Molar mass | 203.237 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
| | |
Medical use
is indicated for the control of absence seizures that are refractory to other drugs.[2]
References
- Pfizer AG (2005). "Petinutin (Mésuximide)". Official Pfizer AG Website (in French). Archived from the original on April 22, 2005. Retrieved August 21, 2006.
- Pfizer Inc. (2008). "Celontin (methsuximide capsules, USP)". Official Pfizer Inc. Website. Retrieved November 21, 2014.
- Porter RJ, Penry JK, Lacy JR, Newmark ME, Kupferberg HJ. Plasma concentrations of phensuximide, methosuximide, and their metabolites in relation to clinical efficacy. Neurology 29: 1509-1513, 1979.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.