Bimatoprost
Bimatoprost, sold under the trade name Lumigan among others, is a medication used to treat high pressure inside the eye including glaucoma.[1] Specifically it is used for open angle glaucoma when other agents are not sufficient.[2][1] It may also be used to increase the size of the eyelashes.[3] It is used as an eye drop and effects generally occur within 4 hours.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Lumigan, Latisse, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602030 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Topical (eye drops) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Low |
| Protein binding | 88% |
| Onset of action | 4 hrs |
| Elimination half-life | 45 min after IV application |
| Duration of action | ≥ 24 hrs |
| Excretion | 67% kidney, 25% fecal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.170.712 |
| Chemical and physical data | |
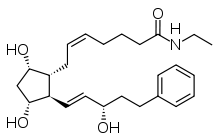
| Formula | C25H37NO4 |
| Molar mass | 415.566 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Common side effects include red eyes, dry eyes, change in color of the eyes, blurry vision, and cataracts.[1][2] Use during pregnancy or breastfeeding is generally not recommended.[2] It is a prostaglandin analog and works by increasing the outflow of aqueous fluid from the eyes.[1]
Bimatoprost was approved for medical use in the United States in 2001.[1] It is available as a generic medication.[2] A 3 milliliter bottle in the United Kingdom costs the NHS about 9.30 £ as of 2019.[2] In the United States the wholesale cost of this amount is about US$80.[4] In 2016, it was the 254th most prescribed medication in the United States with more than a million prescriptions.[5]
Uses
Medical
Bimatoprost is used for the treatment of open-angle glaucoma and ocular hypertension in adult patients, either alone or in combination with a beta blocker[6][7] (typically timolol).
Studies have shown bimatoprost to be more effective than timolol in reduction of intraocular pressure (IOP) and at least as effective as the prostaglandin analogs latanoprost and travoprost in reducing IOP.[8]
Cosmetic
Bimatoprost may be used to treat small or underdeveloped eyelashes.[3] The medical term for this is treatment of hypotrichosis; however, the FDA approval is for purely cosmetic purposes (see Prostaglandin F receptor#Clinical significance).[9]
Side effects
Side effects are similar to other prostaglandin analogs applied to the eye. The most common one is conjunctival hyperemia, which occurs in more than 10% of patients. Other effects include blurred vision, eye and eyelid redness, eye burning or other discomfort, and permanent darkening of the iris to brown.[6][7][10] Occasional adverse effects (in less than 1% of patients) are headache and nausea.[6]
Some side effects are specific to the cosmetic formulation, which is applied to the skin at the base of the eyelash rather than instilled into the eye. These include infection if the one-time applicators are reused, and darkening of the eyelid or of the area beneath the eye.[10][11] Research suggests that wiping the eye with an absorbent pad after the administration of eye drops can result in shorter eyelashes and a lesser chance of hyperpigmentation in the eyelid, compared to not wiping off excess fluid.[12]
Interactions
No interaction studies with this substance have been performed. Interactions with systemic (for example, oral) drugs are considered unlikely because bimatoprost does not reach relevant concentrations in the bloodstream. Bimatoprost does not negatively interact with timolol eye drops.[6]
Pharmacology
Mechanism of action
Bimatoprost is a structural analog of prostaglandin F2α (PGF2α). Like other PGF2α analogs such as travoprost, latanoprost and tafluprost, it increases the outflow of aqueous fluid from the eye and lowers intraocular pressure. However, in contrast to these it does not act on the prostaglandin F receptor, nor on any other known prostaglandin receptor. It is thought that bimatoprost mimics the human body's own prostamides (which are chemically similar), a class of substances related to prostaglandins, but with an unknown mechanism of action.[6][7] No prostamide receptor has been identified as of 2015; the search is ongoing.[13] As of 2019 it was thought that bimatoprost worked via the trabecular meshwork and uveoscleral pathways.[14][15]
Pharmacokinetics
Bimatoprost is well absorbed through the cornea. It starts lowering intraocular pressure after four hours, lasting for at least 24 hours. A low percentage enters the bloodstream. In the blood plasma, peak concentrations are reached after 10 minutes, then drop below the detection limit of 25 pg/ml after 1.5 hours. The substance does not accumulate in the body.[6][7]
Plasma protein binding is 88%. Bimatoprost is metabolized by oxidation, N-deethylation and glucuronidation, forming a variety of metabolites. Biological half-life was measured to be 45 minutes after intravenous infusion. 67% are eliminated via the kidney, and 25% via the feces.[6][7]
References
- "Bimatoprost Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 26 March 2019.
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 1149. ISBN 9780857113382.
- "Bimatoprost ophthalmic solution" (PDF). Food and Drug Administration (FDA). Retrieved 26 March 2019.
- "NADAC as of 2019-02-27". Centers for Medicare & Medicaid Services (CMS). Retrieved 3 March 2019.
- "The Top 300 of 2019". clincalc.com. Retrieved 22 December 2018.
- Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
- Drugs.com: Bimatoprost Monograph.
- Curran MP (2009). "Bimatoprost: a review of its use in open-angle glaucoma and ocular hypertension". Drugs Aging. 26 (12): 1049–71. doi:10.2165/11203210-000000000-00000. PMID 19929032.
- Choi YM, Diehl J, Levins PC (2015). "Promising alternative clinical uses of prostaglandin F2α analogs: beyond the eyelashes". Journal of the American Academy of Dermatology. 72 (4): 712–6. doi:10.1016/j.jaad.2014.10.012. PMID 25601618.
- Latisse prescribing information
- Louis, Catherine Saint (1 May 2010). "Long Lashes Without Prescription, but With Risks". The New York Times. Retrieved 18 July 2019.
- Xu L, Wang X, Wu M (2017). "Topical medication instillation techniques for glaucoma". Cochrane Database Syst Rev. 2: CD010520. doi:10.1002/14651858.CD010520.pub2. PMC 5419432. PMID 28218404.
- Shelnut, E. L.; Nikas, S. P.; Finnegan, D. F.; Chiang, N; Serhan, C. N.; Makriyannis, A (2015). "Design and synthesis of novel prostaglandin E2 ethanolamide and glycerol ester probes for the putative prostamide receptor(s)". Tetrahedron Letters. 56 (11): 1411–1415. doi:10.1016/j.tetlet.2015.01.164. PMC 4422110. PMID 25960577.
- "Australian Product Information Lumigan (Bimatoprost) Eye Drops" (PDF). Retrieved 15 July 2019.
Bimatoprost reduces intraocular pressure in man by increasing aqueous humour outflow through the trabecular meshwork and enhancing uveoscleral outflow.
- "Bimatoprost". www.drugbank.ca. Retrieved 7 July 2019.