Gosogliptin
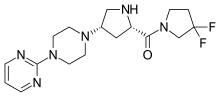
Gosogliptin (INN; trade name SatRx) is a drug for the treatment of type II diabetes. It is in the class of dipeptidyl peptidase-4 (DPP-4) inhibitors (also called gliptins). It was discovered[1] and developed through Phase 2 by Pfizer.[2] It is approved for use in Russia.[3]
 | |
| Clinical data | |
|---|---|
| Other names | PF-734,200 |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C17H24F2N6O |
| Molar mass | 366.417 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Ammirati MJ, Andrews KM, Boyer DD, Brodeur AM, Danley DE, Doran SD, et al. (April 2009). "(3,3-Difluoro-pyrrolidin-1-yl)-[(2S,4S)-(4-(4-pyrimidin-2-yl-piperazin-1-yl)-pyrrolidin-2-yl]-methanone: a potent, selective, orally active dipeptidyl peptidase IV inhibitor". Bioorganic & Medicinal Chemistry Letters. 19 (7): 1991–5. doi:10.1016/j.bmcl.2009.02.041. PMID 19275964.
- Dai H, Johnson SL, Terra SG, Marbury TC, Smith WB, Alcorn H, Boyd RA, Wang R, Nguyen TT (July 2011). "The pharmacokinetics of PF-734200, a DPP-IV inhibitor, in subjects with renal insufficiency". British Journal of Clinical Pharmacology. 72 (1): 85–91. doi:10.1111/j.1365-2125.2011.03954.x. PMC 3141189. PMID 21366665.
- "SatRx LLC Announces First Registration in Russia of SatRx (gosogliptin), an Innovative Drug for Treatment of Type 2 Diabetes" (Press release). SatRx LLC.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.