Dapagliflozin
Dapagliflozin, sold under the brand name Farxiga among others, is a medication used to treat type 2 diabetes. It is of the gliflozin class. It was developed by Bristol-Myers Squibb in partnership with AstraZeneca.
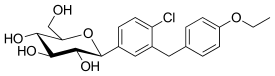 | |
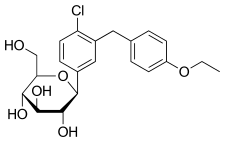 Haworth projection (bottom) | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌdæpəɡlɪˈfloʊzɪn/ DAP-ə-glif-LOH-zin |
| Trade names | Forxiga, Farxiga, others |
| Other names | BMS-512148; (1S)-1,5-anhydro-1-C-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-D-glucitol |
| AHFS/Drugs.com | UK Drug Information |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 78% (after 10 mg dose) |
| Protein binding | ~91% |
| Metabolism | UGT1A9 (major), CYP (minor) |
| Metabolites | Dapagliflozin 3-O-glucuronide (inactive) |
| Elimination half-life | ~12.9 hours |
| Excretion | Urine (75%), feces (21%)[1]:5 |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.167.331 |
| Chemical and physical data | |
| Formula | C21H25ClO6 |
| Molar mass | 408.873 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Medical uses
Dapagliflozin is used along with diet and exercise to improve glycemic control in adults with type 2 diabetes.[2] SGLT2 inhibitors, including dapagliflozin, reduce the likelihood of hospitalization for congestive heart failure or progression of renal disease in persons with diabetes mellitus type 2 and reduce the likelihood of stroke and heart attack in persons with diabetes mellitus type 2 who have known atherosclerotic vascular disease.[3] In patients having heart failure with a reduced ejection fraction, treatment with dapagliflozin has shown to reduce the risk of worsening of heart failure or progression to death from cardiovascular causes, irrespective of patients diabetic status.[4]
In 2012, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency issued a positive opinion on the drug.[5] It is now marketed in a number of European countries.[6]
Adverse effects
Since dapagliflozin leads to heavy glycosuria (sometimes up to about 70 grams per day) it can lead to rapid weight loss and tiredness. The glucose acts as an osmotic diuretic (this effect is the cause of polyuria in diabetes) which can lead to dehydration. The increased amount of glucose in the urine can also worsen the infections already associated with diabetes, particularly urinary tract infections and thrush (candidiasis). Rarely, use of a SGLT2 drug, including dapagliflozin, is associated with necrotizing fasciitis of the perineum, also called Fournier gangrene.[7]
Dapagliflozin is also associated with hypotensive reactions. There are concerns it may increase the risk of diabetic ketoacidosis.[8]
Mechanism of action
Dapagliflozin inhibits subtype 2 of the sodium-glucose transport proteins (SGLT2) which are responsible for at least 90% of the glucose reabsorption in the kidney. Blocking this transporter mechanism causes blood glucose to be eliminated through the urine.[9] In clinical trials, dapagliflozin lowered HbA1c by 0.6 versus placebo percentage points when added to metformin.[10]
Regarding its protective effects in heart failure, this is attributed primarily to haemodynamic effects, where SGLT2 inhibitors potently reduce intravascular volume through osmotic diuresis and natriuresis. This consequently may lead to a reduction in preload and afterload, thereby alleviating cardiac workload and improving left ventricular function.[11]
Names
Dapagliflozin is the INN,[13] and USAN.[14]
There is a combination product dapagliflozin/metformin extended-release, called Xigduo XR.[15]
In Feb 2017 the FDA approved a once-daily combination of dapagliflozin and saxagliptin, as Qtern.[16]
Research
Clinical trials to assess effectiveness for patients with type 1 diabetes are underway.[17][18]
References
- "Farxiga (dapagliflozin) Tablets, for Oral Use. Full Prescribing Information" (PDF). AstraZeneca Pharmaceuticals. Retrieved 15 November 2016.
- "FDA Approves Farxiga to Treat Type 2 Diabetes". Food and Drug Administration. 8 January 2014. Retrieved 15 November 2016.
- Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS (January 2019). "SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials". Lancet. 393 (10166): 31–39. doi:10.1016/S0140-6736(18)32590-X. PMID 30424892.
- McMurray, John J.V.; Solomon, Scott D.; Inzucchi, Silvio E.; Køber, Lars; Kosiborod, Mikhail N.; Martinez, Felipe A.; Ponikowski, Piotr; Sabatine, Marc S.; Anand, Inder S.; Bělohlávek, Jan; Böhm, Michael; Chiang, Chern-En; Chopra, Vijay K.; de Boer, Rudolf A.; Desai, Akshay S.; Diez, Mirta; Drozdz, Jaroslaw; Dukát, Andrej; Ge, Junbo; Howlett, Jonathan G.; Katova, Tzvetana; Kitakaze, Masafumi; Ljungman, Charlotta E.A.; Merkely, Béla; Nicolau, Jose C.; O’Meara, Eileen; Petrie, Mark C.; Vinh, Pham N.; Schou, Morten; Tereshchenko, Sergey; Verma, Subodh; Held, Claes; DeMets, David L.; Docherty, Kieran F.; Jhund, Pardeep S.; Bengtsson, Olof; Sjöstrand, Mikaela; Langkilde, Anna-Maria (19 September 2019). "Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction". New England Journal of Medicine. doi:10.1056/NEJMoa1911303.
- "Forxiga EPAR summary for the public" (PDF). European Medicines Agency. 12 November 2012.
- Drugs.com: International Drug Names for Forxiga.
- Research, Center for Drug Evaluation and (9 February 2019). "FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes". FDA – via www.fda.gov.
- "Safety Alerts for Human Medical Products — SGLT2 inhibitors: Drug Safety Communication — FDA Warns Medicines May Result in a Serious Condition of Too Much Acid in the Blood". Food and Drug Administration. 15 May 2015. Retrieved 15 November 2016.
- "Life Sciences - Clarivate". Clarivate.
- "UEndocrine: Internet Endocrinology Community". uendocrine.com.
- "The effects of sodium-glucose cotransporter 2 inhibitors on left ventricular function: current evidence and future directions".
- Schubert-Zsilavecz, M, Wurglics, M, Neue Arzneimittel 2008/2009
- "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 59" (PDF). World Health Organization. 2008. p. 50. Retrieved 15 November 2016.
- "Statement on a Nonproprietary Name Adopted by the USAN Council" (PDF). American Medical Association. Archived from the original (PDF) on 7 February 2012. Retrieved 15 November 2016.
- "US FDA Approves Once-Daily Xigduo™ XR Tablets for Adults with Type 2 Diabetes". www.astrazeneca.com. AstraZeneca. 30 October 2014.
- "Efficacy and Safety of Dapagliflozin, Added to Therapy of Patients With Type 2 Diabetes With Inadequate Glycemic Control on Insulin - Full Text View - ClinicalTrials.gov". clinicaltrials.gov.
- "Bristol-Myers Squibb - Our Company". ctr.bms.com. Archived from the original on 2011-07-08. Retrieved 2009-05-18.