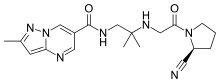
Anagliptin
Anagliptin (INN; trade name Suiny) is a pharmaceutical drug for the treatment of type 2 diabetes mellitus. It is approved for use in Japan.[1] It belongs to the class of anti-diabetic drugs known as dipeptidyl peptidase-4 inhibitors or "gliptins".[2]
 | |
| Clinical data | |
|---|---|
| Trade names | Suiny |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C19H25N7O2 |
| Molar mass | 383.45 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- A.I. Graul; B. Lupone; E. Cruces; M. Stringer (2013). "2012 in Review - Part I: The Year's New Drugs and Biologics" (PDF). Drugs of Today. 49 (1): 33–68. doi:10.1358/dot.2013.49.1.1933991. Archived from the original (PDF) on 2013-11-03.
- Kato, Noriyasu; Oka, Mitsuru; Murase, Takayo; Yoshida, Masahiro; Sakairi, Masao; Yamashita, Satoko; Yasuda, Yoshika; Yoshikawa, Aya; Hayashi, Yuuji; Makino, Mitsuhiro; Takeda, Motohiro; Mirensha, Yakufu; Kakigami, Takuji (2011). "Discovery and pharmacological characterization of N-\2-({2-\(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl}amino)-2-methylpropyl]-2-methylpyrazolo\1,5-a]pyrimidine-6-carboxamide hydrochloride (anagliptin hydrochloride salt) as a potent and selective DPP-IV inhibitor". Bioorganic & Medicinal Chemistry. 19 (23): 7221. doi:10.1016/j.bmc.2011.09.043.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.