Tesaglitazar
Tesaglitazar (also known as AZ 242) is a dual peroxisome proliferator-activated receptor agonist with affinity to PPARα and PPARγ, proposed for the management of type 2 diabetes.[1]
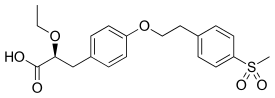 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.201.079 |
| Chemical and physical data | |
| Formula | C20H24O6S |
| Molar mass | 392,47 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
The drug had completed several phase III clinical trials,[2] however in May, 2006 AstraZeneca announced that it had discontinued further development.[3]
Cardiac toxicity of tesaglitazar is related mitochondrial toxicity caused by decrease in PPARγ coactivator 1-α (PPARGC1A, PGC1α) and sirtuin 1 (SIRT1).[4]
References
- Wilding JP, Gause-Nilsson I, Persson A (2007). "Tesaglitazar, as add-on therapy to sulphonylurea, dose-dependently improves glucose and lipid abnormalities in patients with type 2 diabetes". Diab Vasc Dis Res. 4 (3): 194–203. doi:10.3132/dvdr.2007.040. PMID 17907109.
- "GALIDA (tesaglitazar) Clinical Trial Report Summaries". AstraZeneca. Retrieved 2008-03-17.
- "AstraZeneca Discontinues Development of GALIDA (tesaglitazar)". AstraZeneca. 2006-05-04. Retrieved 2012-07-23.
- Kalliora, Charikleia; Kyriazis, Ioannis D.; Oka, Shin-ichi; Lieu, Melissa J.; Yue, Yujia; Area-Gomez, Estela; Pol, Christine J.; Tian, Ying; Mizushima, Wataru; Chin, Adave; Scerbo, Diego; Schulze, P. Christian; Civelek, Mete; Sadoshima, Junichi; Madesh, Muniswamy; Goldberg, Ira J.; Drosatos, Konstantinos (2019). "Dual PPARα/γ activation inhibits SIRT1-PGC1α axis and causes cardiac dysfunction". JCI Insight. 4 (17). doi:10.1172/jci.insight.129556. ISSN 2379-3708.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.