Suprofen
Suprofen is a nonsteroidal anti-inflammatory drug (NSAID) developed by Janssen Pharmaceutica[1] that was marketed as 1% eye drops under the trade name Profenal.
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | Oral, eye drops |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 20% |
| Identifiers | |
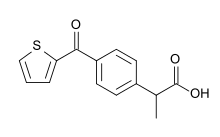
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.050.071 |
| Chemical and physical data | |
| Formula | C14H12O3S |
| Molar mass | 260.309 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Uses
Suprofen was originally used as tablet, but oral uses have been discontinued due to renal effects.[2] It was subsequently used exclusively as a topical ophthalmic solution, typically to prevent miosis during and after ophthalmic surgery.[3] This application has been discontinued as well, at least in the US.[4]
References
- Janssen, Paul A.; Van Daele, Georges H. P.; Boey, Jozef M. "Antiphlogistic aroyl-substituted phenylacetic acid derivatives" (1974) DE 2353357
- Nies A S (1988). "Renal effects of nonsteroidal anti-inflammatory drugs". Agents and Actions. 24: 95–106. doi:10.1007/978-3-0348-9160-8_9. ISBN 978-3-0348-9931-4. PMID 3142236.
- Guidance for FDA Staff and Industry Compliance Policy Guides Manual, Sec. 460.200
- Drugs.com: suprofen ophthalmic
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.