Funapide
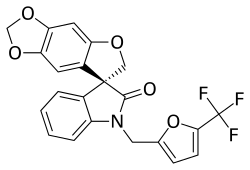
Funapide (INN) (former developmental code names TV-45070 and XEN402) is a novel analgesic under development by Xenon Pharmaceuticals (formerly in partnership with Teva Pharmaceutical Industries) for the treatment of a variety of chronic pain conditions, including osteoarthritis, neuropathic pain, postherpetic neuralgia, and erythromelalgia, as well as dental pain.[1][2][3][4] It acts as a small-molecule Nav1.7 and Nav1.8 voltage-gated sodium channel blocker.[1][2][4] Funapide is being evaluated in humans in both oral and topical formulations, and as of July 2014, has reached phase IIb clinical trials.[1][3]
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth, topical |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C22H14F3NO5 |
| Molar mass | 429.34547 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- Bagal, Sharan K.; Chapman, Mark L.; Marron, Brian E.; Prime, Rebecca; Ian Storer, R.; Swain, Nigel A. (2014). "Recent progress in sodium channel modulators for pain". Bioorganic & Medicinal Chemistry Letters. 24 (16): 3690–9. doi:10.1016/j.bmcl.2014.06.038. ISSN 0960-894X. PMID 25060923.
- Stephen McMahon; Martin Koltzenburg; Irene Tracey; Dennis C. Turk (1 March 2013). Wall & Melzack's Textbook of Pain: Expert Consult - Online. Elsevier Health Sciences. p. 508. ISBN 0-7020-5374-0.
- Xenon Pharma. "TV-45070: A Small Molecule for the Treatment of the Orphan Disease EM and Other Pain Disorders".
- Xenon Pharma (2012). "Teva and Xenon Announce Teva's World Wide License of Xenon's Pain Drug XEN402".
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.