Dextromoramide
Dextromoramide[3](Palfium, Palphium, Jetrium, Dimorlin)[4] is a powerful opioid analgesic approximately three times more potent than morphine but shorter acting.[5] It is subject to drug prohibition regimes, both internationally through UN treaties and by the criminal law of individual states. It is still marketed solely in the Netherlands.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Palfium |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral, Rectal, Intravenous, Insufflation[1] |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | >75%[2] |
| Protein binding | High[2] |
| Metabolism | Liver, partly mediated by CYP3A4[2] |
| Elimination half-life | 3.5 hours[2] |
| Excretion | Urine, faeces[2] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.013 |
| Chemical and physical data | |
| Formula | C25H32N2O2 |
| Molar mass | 392.534 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
History
Dextromoramide was discovered and patented in 1956 by Dr Paul Janssen at Janssen Pharmaceutica, who also discovered fentanyl, another important synthetic opioid, widely used to treat pain and in combination with other drugs as an anaesthetic, as well as haloperidol, piritramide, the loperamide-diphenoxylate series and other important drugs[6]
Dextromoramide was singled out along with ketobemidone and several other synthetics by the United Nations and European Union as being "extra-dangerous" in the early 1960s, with dextromoramide being alleged to be three times more euphoric than heroin at equianalgesic doses, though this did little to stem production in the first half of the decade. The development of the moramides and the coming to fruition of work on piritramide were two of the events that precipitated the 1961 update to the Single Convention On Narcotic Drugs, as cited by Dr Shulgin in Controlled Substances and various monographs.
Dextromoramide was much favoured by drug users in Australia in the 1970s and the United Kingdom.[7] It has the main proprietary name of Palfium amongst others, though as of mid-2004 the drug was discontinued in the UK due to limited supplies of precursor chemicals. Although this is true, it is believed there was an approximate one year shortage of Dextromoramide and the real reason that Palfium was not put back into production for the UK market is because of how addictive and potent it is as an oral painkiller. Dependence liability is similar to morphine, but with a less severe withdrawal syndrome.
The only European countries that now use the brand Palfium are the Netherlands, Ireland and Luxembourg. It is presumably able to be imported into other Schengen zone countries under Title 76 of said treaty coming into force in 2002. It is a Schedule I Narcotic controlled substance in the United States, with a DEA ACSCN of 9613 and an annual aggregate manufacturing quota of zero as of 2013, and had been out of use in the United States for around a decade when the new Controlled Substances Act 1970 was promulgated. The salts of dextromoramide in use are the hydrochloride (free base conversion ratio 0.915) and tartrate (0.724).[8] Racemoramide and moramide intermediate are also controlled.
Medical use
The main advantage of this drug is that it has a fast onset of action when taken orally, and has a high bioavailability which means that oral dosing produces almost as much effect as injection. It also has a relatively low tendency to cause constipation which is a common problem with opioid analgesics used for cancer pain relief, and tolerance to the analgesic effects develops relatively slowly compared to most other short-acting opioids.[9]
Pharmacokinetics
Dextromoramide has a mean elimination half life of 215.3 +/- 78.4 minutes and volume of distribution of 0.58 +/- 0.20 L/kg.[10] Peak plasma levels are reached within 0.5-4.0 h after dosing, decline of plasma concentrations after the peak follow a biphasic pattern, with half-lives of 0.4-1.6 h for the first phase and 6.3-21.8 h for the terminal phase. While in about 40% of patients, half-lives from 1.5 to 4.7 h, in a monophasic manner. Less than 0.06% of the dose is excreted unchanged in urine within 8 h of administration.[11]
Chemistry
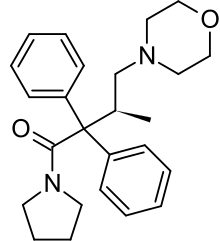
Dextromoramide is the right-handed isomer of the moramide molecule. The left-handed molecule is called levomoramide, and a mixture of the two is called racemoramide. Its full chemical name is (+)-1-(3-Methyl-4-morpholino-2,2-diphenylbutyryl)pyrrolidine, and its molecular formula: C25H32N2O2, with an atomic weight of ~392.5.
Dextromoramide was discovered during the course of research into a related family of compounds, the α,α-Diphenyl-γ-Dialkyamino-Butyramides, which show no analgesic activity, but are extremely active physiologically as inhibitors of gastric secretions in man. Other drugs from this series show antispasmodic and antihistamine effects, but most research was put into researching analgesics.
The structure-activity relationships of this family of drugs was investigated extensively, with dextromoramide representing the optimisation of several different structural features;
(i) at the 1-amide group only the pyrrolidine and dimethylamide substituents were active, with pyrrolidine being more potent
(ii) the alkyl chain was more potent when methylated, 3-methylation was more potent than 4-methylation, and in the 3-methyl analogues the dextro isomer was more active
(iii) while morpholine, dimethylamine, pyrrolidine and piperidine were all active at the 4-amine group, morpholine was the most active
(iv) any substitution on the phenyl rings reduces activity.
So dextromoramide, with a pyrrolidine ring on the 1-amide position, a dextro methyl group on the 3-position of the alkyl chain, a morpholine ring around the 4-amine group, and both phenyl rings unsubstituted, was by far the most potent out of all the compounds in this series and was the only one that became widely used in medicine (although the racemic mix racemoramide saw some limited use).[12]
References
- Brayfield A, ed. (13 December 2013). "Dextromoramide". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 23 April 2014.
- "SAMENVATTING VAN DE PRODUCTKENMERKEN" (PDF). ACE Pharmaceuticals website (in Dutch). ACE Pharmaceuticals. January 2002. Retrieved 23 April 2014.
- GB Patent 822055 Improvements in or relating to new analgesically active substituted alpha, alpha-diphenyl--y-amino-butyramides and the manufacture thereof
- GB Patent 822055
- Twycross RG, Lack SA (1989). Oral morphine in advanced cancer (2nd ed.). Beaconsfield. ISBN 978-0-906584-27-9.
- López-Muñoz F, Alamo C (April 2009). "The consolidation of neuroleptic therapy: Janssen, the discovery of haloperidol and its introduction into clinical practice". Brain Research Bulletin. 79 (2): 130–41. doi:10.1016/j.brainresbull.2009.01.005. PMID 19186209.
- Newgreen DB (1980). "Dextromoramide: Review and Case Report". Australian Journal of Pharmacy. 61: 641–44.
- "Conversion Factors for Controlled Substances". Drug Enforcement Administration. U.S. Department of Justice.
- de Vos JW, Ufkes JG, van den Brink W, van Brussel GH, de Wolff FA (1999). "Craving patterns in methadone maintenance treatment with dextromoramide as adjuvant". Addictive Behaviors. 24 (5): 707–13. doi:10.1016/s0306-4603(98)00081-1. PMID 10574310.
- Lançon JP, Pechinot A, Athis PD, Pechinot M, Pointaire P, Obadia JF, Caillard B (1989). "[Pharmacokinetics of dextromoramide in the surgical patient]". Annales Francaises d'Anesthesie et de Reanimation. 8 (5): 488–92. doi:10.1016/s0750-7658(89)80015-2. PMID 2576345.
- Pagani I, Barzaghi N, Crema F, Perucca E, Ego D, Rovei V (1989). "Pharmacokinetics of dextromoramide in surgical patients". Fundamental & Clinical Pharmacology. 3 (1): 27–35. doi:10.1111/j.1472-8206.1989.tb00027.x. PMID 2714730.
- Janssen PA (1960). Synthetic Analgesics Part 1: Diphenylpropylamines. Pergamon Press. pp. 141–145.
Further reading
- Den Otter G (August 1958). "[Pain control with dextromoramide (pyrrolamidolum, R 875, palfium)]". Nederlands Tijdschrift voor Geneeskunde (in Dutch; Flemish). 102 (34): 1637–41. PMID 13590312.CS1 maint: unrecognized language (link)
- Lasagna L, De Kornfeld T, Safar P (December 1958). "A clinical trial of dextromoramide, (R 875, SKF D-5137)". Journal of Chronic Diseases. 8 (6): 689–93. doi:10.1016/0021-9681(58)90124-3. PMID 13598780.