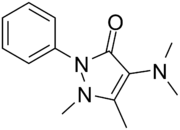
Aminophenazone
Aminophenazone (or aminopyrine, amidopyrine, Pyramidon) is a pyrazolone with analgesic, anti-inflammatory, and antipyretic properties but has risk of agranulocytosis. A breath test with 13C-labeled aminopyrine has been used as a non-invasive measure of cytochrome P-450 metabolic activity in liver function tests.[1] It is also used in measuring the total body water in the human body system.[2] Production and use have been banned in France, Thailand and India.
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | N-demethylation[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.332 |
| Chemical and physical data | |
| Formula | C13H17N3O |
| Molar mass | 231.29358 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
History
Aminophenazone was first synthesized by Friedrich Stolz and Ludwig Knorr in the late nineteenth century, and sold as an anti-fever medicine known as Pyramidon by Hoechst AG from 1897 until its eventual replacement by the safer propyphenazone molecule.
Symptoms
Symptoms of exposure to this compound include:[3]
- allergic reactions
- strong spasmolytic effect on smooth muscle of peripheral blood vessels
- irritability
- palsy
- copious sweating
- dilated pupils
- sharp drop then rise in body temperature
- dysuria
- dyspnea
- anxiety
- rectal tenesmus
- urinary frequency
- intermittent fever
- fatty infiltration of the liver
- heart muscle degeneration
- death due to circulatory failure following cardiovascular collapse
Agranulocytosis often occurs. Ingestion may cause central nervous system stimulation, vomiting, convulsions, cyanosis, tinnitus, leukopenia, kidney damage and coma. Ingestion may also lead to nausea, mental disturbances, methemoglobinemia, chocolate-colored blood, dizziness, epigastric pain, difficulty in hearing, thready pulse and liver damage.
Other symptoms reported via ingestion include hemolytic anemia, porphyria and severe gastrointestinal bleeding. Bone marrow depression also occurs. Rare eye effects include acute transient myopia.
Chronic symptoms include:
- anorexia
- edema
- oliguria
- urticaria
- hypersensitivity
- aplastic anemia
- sore throat
- fever
- pharyngeal membrane
- jaundice enlargement of the liver and spleen
- exfoliative dermatitis
- gastric or duodenal erosion with perforation or bleeding
- adrenal necrosis
- thrombocytopenic purpura
- acute leukemia
When heated to decomposition this compound emits toxic fumes of nitrogen oxides.
Metabolism
Amidopyrine is metabolized by demethylation and acetylation. Amidopirina metabolites are 4-aminoantipyrine, metilaminoantipirin, rubazonovaya and metilrubazonovaya acid. These acids have a reddish color. Due to the presence in the urine of these acids makers amidopirina high doses, it can have a reddish brownish coloration.[4]
References
- Caubet, M. S.; Laplante, A.; Caillé, J.; Brazier, J. L. (4 Jan 2007). "[13C]Aminopyrine and [13C]Caffeine Breath Test: Influence of Gender, Cigarette Smoking and Oral Contraceptives Intake". Isotopes in Environmental and Health Studies. 38 (2): 71–77. doi:10.1080/10256010208033314.
- "Aminophenazone — Compound Summary". PubChem. The National Library of Medicine. 2005-03-26. Retrieved June 12, 2008.
- Pubchem. "aminophenazone". pubchem.ncbi.nlm.nih.gov. Retrieved 2017-11-09.
- Vyzanie. "Amidopirina". soundlike.ru. Retrieved 2017-11-09.