Phenazone
Phenazone (INN and BAN; also known as phenazon, antipyrine (USAN), or analgesine) is an analgesic, a nonsteroidal anti-inflammatory drug (NSAID) and an antipyretic.
 | |
| Clinical data | |
|---|---|
| Other names | analgesine, antipyrine |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 12 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.442 |
| Chemical and physical data | |
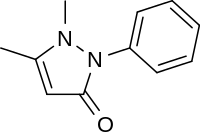
| Formula | C11H12N2O |
| Molar mass | 188.2258g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
History
It was first synthesized by Ludwig Knorr in 1887.[1][2]:26–27 Phenazone is synthesized[3] by condensation of phenylhydrazine and ethyl acetoacetate under basic conditions and methylation of the resulting intermediate compound 1-phenyl-3-methylpyrazolone[4] with dimethyl sulfate or methyl iodide. It crystallizes in needles which melt at 156 °C (313 °F). Potassium permanganate oxidizes it to pyridazine tetracarboxylic acid. Phenazone has an elimination half life of about 12 hours.[5] Indication: Used to relieve pain and fever. Antipyrine is often used in testing the effects of other drugs or diseases on drug-metabolizing enzymes in the liver.[6]
Phenazone was used before 1911 as an analgesic and antipyretic. The dose was 5-20 grams, but on account of its depressant action on the heart, and the toxic effects to which it occasionally gives rise it was replaced by phenacetin.[7]
Preparation
The Encyclopedia Britanica 1911 states that phenazone could be prepared by the condensation of phenylhydrazine with aceto-acetic ester, the resulting phenyl methyl pyrazolone being heated with methyl iodide and methyl alcohol to 100-110 °C.
On the large scale phenylhydrazine is dissolved in dilute sulfuric acid, the solution warmed to about 40 °C and the aceto-acetic ester added. When the reaction is complete the acid is neutralized with soda, and the phenyl methyl pyrazolone extracted with ether and distilled in vacuo. The portion distilling at about 200 °C is then methylated by means of methyl alcohol and methyl iodide at 100-110 °C, the excess of methyl alcohol removed and the product obtained decolorized by sulfuric acid. The residue is treated with a warm concentrated solution of soda, and the oil which separates is removed by shaking with benzene. The benzene layer on evaporation deposits the anti-pyrine as a colourless crystalline solid which melts at 113 °C and is soluble in water. It is basic in character, and gives a red coloration on the addition of ferric chloride. [8]
Adverse effects
Possible adverse effects include:
- Allergy to pyrazolones
- Nausea
- Agranulocytosis
- Hepatotoxicity
See also
- Propyphenazone
- A/B Otic Drops, ear drops combined with benzocaine to relieve pain and remove cerumen
References
- Brune, K (1997). "The early history of non-opioid analgesics". Acute Pain. 1: 33. doi:10.1016/S1366-0071(97)80033-2.
- Enrique Ravina. The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons, 2011 ISBN 9783527326693
- . ISBN 8122415652. Missing or empty
|title=(help):226 - "Chemspider". Chemspider. Retrieved February 24, 2019.
- http://www.mims.com/USA/drug/info/phenazone/?q=Other%20Ear%20Preparations&type=full
- "Antipyrine drugs and health products". sDrugs.com.
-

-

- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 2 (11th ed.). Cambridge University Press. p. 134.