Tilidine
Tilidine, or tilidate (brand names: Tilidin, Valoron and Valtran) is a synthetic opioid painkiller, used mainly in Germany, Switzerland, South Africa and Belgium for treatment of moderate to severe pain, both acute and chronic.[3] Its onset of pain relief after oral administration is about 10–15 minutes and peak relief from pain occurs about 25–50 minutes after oral administration.[2]
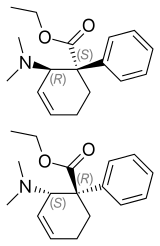 (1S,2R)-tilidine (dextilidine; top), (1R,2S)-tilidine (bottom) | |
| Clinical data | |
|---|---|
| Trade names | Valoron, others |
| Other names | Tilidate (BAN UK) |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, rectal, IM, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 6% (parent compound), 99% (active metabolite)[1] |
| Metabolism | Metabolised by the liver, mostly via the enzymes CYP3A4 and CYP2C19[2] |
| Elimination half-life | 3–5 hours[2] |
| Excretion | Urine (90%)[2] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.037.513 |
| Chemical and physical data | |
| Formula | C17H23NO2 |
| Molar mass | 273.37 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
| | |
Medical uses
Tilidine is used in the form of hydrochloride or phosphate salt. In Germany, tilidine is available in a fixed combination with naloxone for oral administration (Valoron N and generics); the mixture of naloxone is claimed to lower the abuse liability of the opioid analgesic.[2] This is so that if people take the medication orally (which is the way they are meant to) the opioid blocker, naloxone, has minimal effects on them but if they inject it the naloxone becomes bioavailable and hence antagonises the effects of the tilidine producing withdrawal effects.[2][4] In Switzerland the original Valoron brand with only tilidine and no naloxone is also available.[3]
As well as its use as an analgesic, tilidine is also commonly used in Germany for treatment of restless legs syndrome.[5] The reversed ester[6] is also known and is also a prodrug.
Tilidine is a controlled substance in most countries, listed in the German BtMG, Austrian SMG, and in the USA under the Controlled Substances Act 1970 as ACSCN 9750 as a Narcotic under Schedule I, with an annual aggregate manufacturing quota of 10 grams in 2014. It is used as the hydrochloride (free base conversion ratio 0.882) and HCl hemihydrate (0.858) [7]
Adverse effects
Its most common adverse effects are transient nausea and vomiting, dizziness, drowsiness, fatigue, headache and nervousness; less commonly, nausea and vomiting (after repeated dosing), hallucinations, confusion, euphoria, tremor, hyperreflexia, clonus and increased sweating.[2] Uncommonly, somnolence; rarely, diarrhoea and abdominal pain.[2]
Physicochemistry
It usually comes in its hydrochloride hemihydrate salt form; in this form it is highly soluble in water, ethanol and dichloromethane and appears as a white/almost white crystalline powder.[3] Its storage is restricted by its sensitivity to degradation by light and oxygen, hence necessitating its storage in amber bottles and at temperatures below 30 degrees Celsius, respectively.[2][3]
Pharmacology
Considered a low-to-medium-potency opioid, tilidine has the oral potency of about 0.2, that is, a dose of 100 mg p.o. is equianalgesic to approximately 20 mg morphine sulfate orally. It is administered orally (by mouth), rectally (by a suppository), or by injection (SC, IM or slowly IV) with single doses of 50 to 100 mg, the maximal daily dose being up to 600 mg.[8]
Tilidine itself is only a weak opioid, but is rapidly metabolised in the liver and gut to its active metabolite nortilidine and then to bisnortilidine.[9][10] It is the (1S,2R)-isomer (dextilidine)[11] that is responsible for its analgesic activity.[12] The reversed ester of tilidine is known[13]
Tilidine is a prodrug with a weak opioid effect. It is metabolized in the liver to the actual active substance nortilidine. Nortilidin binds to opiate receptors in the central and peripheral nervous systems and suppresses pain perception and transmission.
To counteract the abuse potential, tilidine is used in combination with the opioid receptor antagonist naloxone. Naloxone abolishes the central depressant and peripheral effects of tilidine. The mixing ratio with naloxone is chosen so that the analgesic effect of tilidine is not impaired.
Pharmacokinetics
Tilidine is rapidly absorbed after oral administration and is subject to a pronounced first-pass effect.
The conversion of tilidine into the more active metabolite nortilidine occurs with the participation of CYP3A4 and CYP2C19. The inhibition of these enzymes can thus alter the efficacy and tolerability profile of tilidine. The analgesic effect occurs after 10-15 minutes. After oral administration of 100 mg tilidine plus 8 mg naloxone, the maximum effect is reached in about 25-50 minutes. The duration of action is given as 4-6 hours.
The elimination half-life for nortilidine is 3-5 hours. Tilidine is metabolized to 90% and eliminated renally. The rest appears in the feces.
Depending on the extent of the impairment, the maximum concentration of nortilidine in plasma is lower in liver function than in healthy individuals and the half-life is prolonged. In case of severe hepatic insufficiency the therapy is questionable. In these cases, it is possible that the formation of active nortilidine may be so low that the analgesic effect is insufficient. In addition, in the combination preparations with naloxone, the inactivation of the same can only be insufficient. The consequent antagonization of the nortilidine effect can lead to a further loss of activity [14]
Synthesis
It is manufactured by a Diels-Alder reaction of 1-N,N-dimethylaminobuta-1,3-diene with ethyl atropate, yielding a mixture of isomers,[15] of which only the (E)-(trans)-isomers are active and are separated subsequently from the mixture by precipitation of the inactive (Z)-(cis)-isomers as zinc complex.[9] The inactive (Z)-(cis)-isomers may be epimerized to the more thermodynamically favored (E)-(trans)-isomers via reflux in diluted phosphoric acid.
References
- Vollmer, KO; Thomann, P; Hengy, H (October 1989). "Pharmacokinetics of tilidine and metabolites in man". Arzneimittel-Forschung. 39 (10): 1283–8. PMID 2610722.
- "Tilidin N Sandoz® DP Lösung zum Einnehmen" [Tilidin N Sandoz ® DP oral solution] (PDF) (in German). Wooden Churches: Sandoz Pharmaceuticals GmbH. December 2012. Archived from the original (PDF) on 2 May 2014. Retrieved 18 April 2014.
- Brayfield, A, ed. (13 December 2013). "Tilidine Hydrochloride". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 18 April 2014.
- Brunton, L; Chabner, B; Knollman, B (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill Professional. ISBN 978-0-07-162442-8.
- Tings, T; Trenkwalder, C (2003). "When L-Dopa Preparations, Dopamine Agonists or Opioids? Therapy of Restless Legs Syndrome". MMW Fortschritte der Medizin (in German). 145 (10): 48–49. PMID 12688028.
- US Patent 4921059 Derek. P. Reynolds 'Cycloaliphatic Compoundss Thereof as analgesics' September 22, 1981
- http://www.deadiversion.usdoj.gov/quotas/conv_factor/index.html
- Waldvogel, HH (2001). Analgetika, Antinozizeptiva, Adjuvanzien: Handbuch für die Schmerzpraxis (in German). ISBN 978-3-540-65796-5.
- Buschmann, H (2002). Analgesics: From Chemistry and Pharmacology to Clinical Application. Wiley-VCH. ISBN 978-3-527-30403-5.
- Schulz, R; Bläsig, J; Wüster, M; Herz, A (1978). "The Opiate-Like Action of Tilidine is Mediated by Metabolites". Naunyn-Schmiedeberg's Archives of Pharmacology. 304 (2): 89–93. doi:10.1007/bf00495543. PMID 212687.
- "dextilidine - C17H23NO2 - ChemSpider". www.chemspider.com.
- Drug Discovery and Commercial Exploitation Gerhard Satzinger Drug News Project pages 200–201 14(4) May 2001.
- US 4291059 'Cycloaliphatic Compounds Analgesic possessions Thereof' Derek P. Reynolds
- https://www.gelbe-liste.de/wirkstoffe/Tilidin_18896
- US patent 3557127, Satzinger, G, "Substituted Cyclohexenes, Derivatives thereof and Processes for Obtaining Same", issued 1971-01-19.