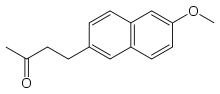
Nabumetone
Nabumetone is a nonsteroidal anti-inflammatory drug (NSAID).[2] Nabumetone has been developed by Beecham. It is available under numerous brand names, such as Relafen, Relifex, and Gambaran.
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692022 |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | > 99% (active metabolite) |
| Metabolism | Hepatic, to active metabolite 6-methoxy-2-naphthylacetic acid; 6-MNA |
| Elimination half-life | 23 hours (active metabolite) |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.169.752 |
| Chemical and physical data | |
| Formula | C15H16O2 |
| Molar mass | 228.29 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Nabumetone is a nonacidic NSAID that is rapidly metabolized in the liver to a major active metabolite, 6-methoxy-2-naphthyl acetic acid. As found with previous NSAIDs, nabumetone's active metabolite inhibits the cyclooxygenase enzyme and preferentially blocks COX-2 activity (which is indirectly responsible for the production of inflammation and pain during arthritis). The active metabolite of nabumetone is felt to be the compound primarily responsible for therapeutic effect. Comparatively, the parent drug is a poor inhibitor of COX-2 byproducts, particularly prostaglandins. It may be less nephrotoxic than indomethacin.[3] There are two known polymorphs of the compound.[4]
Nabumetone has little effect on renal prostaglandin secretion and less of an association with heart failure than other traditional drugs of the class.[5] Effects of nabumetone on blood pressure control in hypertensive patients on ACE inhibitors is also good—equivalent to paracetamol.[6] As of 2015 the cost for a typical month of medication in the United States is 25 to US$50.[7]
Medical uses
Similar in action to other NSAIDs, Nabumetone is used to treat pain and inflammation.
Side effects
It has been shown to have a slightly lower risk of gastrointestinal side effects than most other non-selective NSAIDs since it is a non-acidic prodrug which is then metabolized to its active 6MNA (6-methoxy-2-naphthylacetic acid) form.
Side effects include: Bloody or black, tarry stools; change in color, frequency, or amount of urine; chest pain; shortness of breath; coughing up blood; pale stools; numbness; weakness; flu-like symptoms; leg pain; vision problems; speech problems; problems walking; weight gain; stomach pain; shortness of breath; cold sweat; skin rash; blisters; headache; swelling; bleeding; bruising; vomiting blood; jaundice; diarrhea; constipation; dizziness; indigestion; gas; nausea; and ringing in the ears.[8]
Assay of nabumetone
There are few papers published reporting analytical methods[9] for nabumetone.[10] Two of them employed HPLC with UV-detection.[11][12] One HPLC method using direct injection on restricted access media columns.[13] Flow injection analysis (FIA) with UV-detection was also reported for the determination of nabumetone in pharmaceutical preparations.[14] Methods using HPLC with fluorescence detection [15][16][17][18] were reported. M. Nobilis et al. carried out biotransformation and disposition studies in humans and minipigs using HPLC with UV, fluorescence and mass spectrometric detection. The interactions with gamma-cyclodextrin were also studied by fluorescence measurements. Assay methods employed HPLC using UV detection,[10] photodiode array (PDA) detector[19][20] and mass spectrometric detection for the determination of nabumetone and its metabolites. Murillo Pulgarín et al.[21][22][23] reported three analytical methods using different techniques along with phosphorescence. Liquid chromatography methods using different techniques of mass spectrometry were also reported.[24][25][26] The electrochemical behavior of nabumetone by a voltammetric technique [27] and a novel colorimetric method based on chemical derivatization [28] were also published. P. K. Sahu et al.[29] has reported a HPLC method for simultaneous estimation of nabumetone and paracetamol in combined dosage form.
References
- Varfaj, F.; Zulkifli, S. N. A.; Park, H.-G.; Challinor, V. L.; De Voss, J. J.; Ortiz de Montellano, P. R. (2014-02-28). "Carbon-Carbon Bond Cleavage in Activation of the Prodrug Nabumetone". Drug Metabolism and Disposition. 42 (5): 828–838. doi:10.1124/dmd.114.056903. ISSN 1521-009X. PMC 3989788. PMID 24584631.
- Gonzalo-Garijo MA, Cordobés-Duran C, Lamilla-Yerga AM, Moreno-Gastón I (2007). "Severe immediate reaction to nabumetone". Journal of Investigational Allergology and Clinical Immunology. 17 (4): 274–6. PMID 17694703.
- Olsen, N V; Jensen, N G; Hansen, J M; Christensen, N J; Fogh-Andersen, N; Kanstrup, I L (1999). "Non-steroidal anti-inflammatory drugs and renal response to exercise: a comparison of indomethacin and nabumetone". Clinical Science. 97 (4): 457–465. doi:10.1042/cs0970457.
- Price, C P; Grzesiak, A L; Lang, M; Matzger, A J (2002). "Polymorphism of Nabumetone". Crystal Growth & Design. 2 (6): 501–503. doi:10.1021/cg0255568.
- Donnan, P T (2000). "098. A Drug-Safety Study to Examine the Possible Association of Congestive Heart Failure with Dispensed Nabumetone, Ibuprofen and other Non-Steroidal Anti-inflammatory Drugs". Pharmacoepidemiology & Drug Safety. 8 (S2): S115. doi:10.1002/(SICI)1099-1557(199908)8:2+<S79::AID-PDS429>3.0.CO;2-2.
- Palmer, Robert H; Haig, Ann E; Flavin, Susan K; Iyengar, Malini K (2001). "Effects of ibuprofen (IB), nabumetone (N) and celecoxib (C) on blood pressure (BP) control in hypertensive patients on ACE inhibitors". American Journal of Hypertension. 14 (S1): 85A. doi:10.1016/S0895-7061(01)01811-8.
- Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 9. ISBN 9781284057560.
- "Relafen (Nabumetone): Side Effects, Interactions, Warning, Dosage & Uses". RxList. Retrieved 2018-03-09.
- Małgorzata, Starek; Jan, Krzek (2009). "A review of analytical techniques for determination of oxicams, nimesulide and nabumetone". Talanta. 77 (3): 925–942. doi:10.1016/j.talanta.2008.09.022. PMID 19064072.
- Sahu, Prafulla Kumar; Annapurna, M. Mathrusri (2009). "Analysis of Nabumetone in Bulk and Tablet Formulation by a New and Validated Reverse Phase High Performance Liquid Chromatography". e-Journal of Chemistry. 6(S1): S59–S64. ISSN 0973-4945.
- Al-Momani Idrees, F (1997). "Determination of Nabumetone and Its Major Metabolite in Plasma and Tablet Formulations by Reverse-Phase HPLC". Analytical Letters. 1997 (30): 2485–2492. doi:10.1080/00032719708001759.
- Jang, E J; Lee, Y J; Park, M G; Shim, C K (1995). "HPLC Assay of 6-Methoxy-2-Naphthylacetic Acid, a Major Metabolite of Nabumetone, in Human Serum". Analytical Letters. 28 (13): 2379–2389. doi:10.1080/00032719508000379.
- Ahsanul, Haque; Stewart James, T (1999). "Direct injection hplc analysis of some non‐steroidal anti‐inflammatory drugs on restricted access media columns". Biomedical Chromatography. 13: 51–56. doi:10.1002/(sici)1099-0801(199902)13:1<51::aid-bmc814>3.3.co;2-k.
- Can, N O; Tuncel, M; Aboul-Enein, H Y (2003). "Determination of nabumetone in pharmaceutical formulation by flow injection analysis (FIA) with UV-detection". Die Pharmazie. 58 (1): 22–24. PMID 12622247.
- Mikami, E; Goto, T; Ohno, T; Matsumoto, H; Nishida, M (2000). "Simultaneous analysis of naproxen, nabumetone and its major metabolite 6-methoxy-2-naphthylacetic acid in pharmaceuticals and human urine by high-performance liquid chromatography". Journal of Pharmaceutical and Biomedical Analysis. 23 (5): 917–925. doi:10.1016/s0731-7085(00)00365-4. PMID 11022916.
- Kobylińska, Kamila; Barlińska, Małgorzata; Kobylińska, Maria (2003). "Analysis of nabumetone in human plasma by HPLC. Application to single dose pharmacokinetic studies". Journal of Pharmaceutical and Biomedical Analysis. 2003 (32): 323–328. doi:10.1016/S0731-7085(03)00078-5. PMID 12763542.
- Nobilis, M; Kopecký, J; Kv, Tina J; Svoboda, Z; Pour, M; Kune, J; Hol, Apek M; Kolá, Ová L (2003). "Comparative biotransformation and disposition studies of nabumetone in humans and minipigs using high-performance liquid chromatography with ultraviolet, fluorescence and mass spectrometric detection". Journal of Pharmaceutical and Biomedical Analysis. 32 (4–5): 641–656. doi:10.1016/s0731-7085(03)00171-7. PMID 12899954.
- Al-Rawashdeh, A F Nathir (2005). "Interactions of Nabumetone with γ-Cyclodextrin Studied by Fluorescence Measurements". Journal of Inclusion Phenomena and Macrocyclic Chemistry. 51 (1–2): 27–32. doi:10.1007/s10847-004-1502-9.
- Nageswara, Rao R; Meena, S; Nagaraju, D; Raghu Ram, Rao A (2004). "Development and validation of a reversed-phase liquid chromatographic method for separation and simultaneous determination of COX-2 inhibitors in pharmaceuticals and its application to biological fluids". Biomedical Chromatography. 19 (5): 362–368. doi:10.1002/bmc.458. PMID 15627281.
- Nobilis, M; Holcapek, M; Kolárová, L; Kopecký, J; Kunes, M; Svoboda, Z; Kvetina, J (2004). "Identification and determination of phase II nabumetone metabolites by high-performance liquid chromatography with photodiode array and mass spectrometric detection". Journal of Chromatography A. 1031 (1–2): 229–236. doi:10.1016/j.chroma.2004.01.031. PMID 15058587.
- Murillo, Pulgarín J A; Alañón, Molina A; Alañón, Pardo M T (2005). "Simplex optimization and kinetic determination of nabumetone in pharmaceutical preparations by micellar—stabilized room temperature phosphorescence". Analytica Chimica Acta. 528: 77–82. doi:10.1016/j.aca.2004.10.014.
- Murillo, Pulgarín J A; Alañón, Molina A; Alañón, Pardo M T (2005). "Simplex optimization of the variables affecting the micelle-stabilized room temperature phosphorescence of 6-methoxy-2-naphthylacetic acid and its kinetic determination in human urine". Analytical Biochemistry. 339 (1): 157–164. doi:10.1016/j.ab.2005.01.012. PMID 15766723.
- Pulgarín; Murillo, Jose A; Aurelia Alañón, Molina; Robles Ignacio, Sánchez-Ferrer (2005). "Simple and rapid determination of the active metabolite of nabumetone in biological fluids by heavy atom-induced room temperature phosphorescence". Analytica Chimica Acta. 554 (1–2): 37–42. doi:10.1016/j.aca.2005.08.040.
- Patel Bhavin, N; Naveen, Sharma; Mallika, Sanyal; Arpana, Prasad; Shrivastav Pranav, S (2008). "High-throughput LC-MS/MS assay for 6-methoxy-2-naphthylacetic acid, an active metabolite of nabumetone in human plasma and its application to bioequivalence study". Biomedical Chromatography. 22 (11): 1213–1224. doi:10.1002/bmc.1047. PMID 18651608.
- Wolff, J C; Hawtin, P N; Monté, S; Balogh, M; Jones, T (2001). "The use of particle beam mass spectrometry for the measurement of impurities in a nabumetone drug substance, not easily amenable to atmospheric pressure ionisation techniques". Rapid Communications in Mass Spectrometry. 15 (4): 265–272. Bibcode:2001RCMS...15..265W. doi:10.1002/rcm.214. PMID 11223957.
- Sheen, J F; Her, G R (December 2004). "Application of pentafluorophenyl hydrazine derivatives to the analysis of nabumetone and testosterone in human plasma by liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry". Analytical and Bioanalytical Chemistry. 380 (7–8): 891–7. doi:10.1007/s00216-004-2877-6. PMID 15700167.
- Yuksel, Altun; Burcu, Dogan; Ozkan Sibel, A; Bengi, Uslu (2007). "Development and Validation of Voltammetric Techniques for Nabumetone in Pharmaceutical Dosage Form, Human Serum and Urine" (PDF). Acta Chimica Slovenica. 54: 287–294.
- Adegoke, A O; Idowu, S O; Olaniyi, A A (2007). "Novel determination of nabumetone, a cox-2 inhibitor precursor via its 4-carboxyl-2,6-dinitrobenzene diazonium (CDNBD) derived AZO dye". African Journal of Medicine and Medical Sciences. 36 (3): 249–257. PMID 18390065.
- Sahu, Prafulla Kumar; Annapurna, M. Mathrusri; Sahoo, Dillip Kumar (2011). "A Simple and Sensitive HPLC Method for Simultaneous Analysis of Nabumetone and Paracetamol in Pharmaceutical Formulations" (PDF). e-Journal of Chemistry. 8 (S1): S41–S46. doi:10.1155/2011/607069.