Ethylmorphine
Ethylmorphine (also known as codethyline, dionine, and ethyl morphine) is an opioid analgesic and antitussive.[1][2][3][4][5][6]
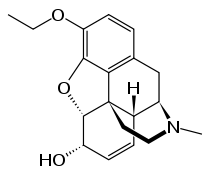 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.883 |
| Chemical and physical data | |
| Formula | C19H23NO3 |
| Molar mass | 313.391 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
See also
References
- Xu, Bang Qian; Aasmundstad, Tor A.; Lillekjendlie, Bjern; Bjørneboe, Anders; Christophersen, Asbjørg S.; Mørland, Jørg (April 1997). "Effects of Ethanol on Ethylmorphine Metabolism in Isolated Rat Hepatocytes: Characterization by Means of a Multicompartmental Model". Pharmacology & Toxicology. 80 (4): 171–181. doi:10.1111/j.1600-0773.1997.tb00392.x. ISSN 1600-0773. PMID 9140136.
- Jonasson, B.; Jonasson, U.; Holmgren, P.; Saldeen, T. (August 1999). "Fatal poisonings where ethylmorphine from antitussive medications contributed to death". International Journal of Legal Medicine. 112 (5): 299–302. doi:10.1007/s004140050253. ISSN 1437-1596. PMID 10460420.
- Popa, Cornelia; Beck, Olof; Brodin, Kerstin (March–April 1998). "Morphine Formation from Ethylmorphine: Implications for Drugs-of-Abuse Testing in Urine". Journal of Analytical Toxicology. 22 (2): 142–147. doi:10.1093/jat/22.2.142. ISSN 1945-2403. PMID 9547411.
- Amacher, David E; Schomaker, Shelli J (31 January 1998). "Ethylmorphine N-demethylase activity as a marker for cytochrome P450 CYP3A activity in rat hepatic microsomes". Toxicology Letters. 94 (2): 115–125. doi:10.1016/S0378-4274(97)00108-2. PMID 9574808.
- Aasmundstad, Ta; Xu, Bq; Johansson, I.; Ripel, A.; Bjorneboe, A.; Christophersen, As; Bodd, E.; Morland, J. (June 1995). "Biotransformation and pharmacokinetics of ethylmorphine after a single oral dose". British Journal of Clinical Pharmacology. 39 (6): 611–620. doi:10.1111/j.1365-2125.1995.tb05720.x. ISSN 1365-2125. PMC 1365072. PMID 7654478.
- Liu, Z; Mortimer, O; Smith, Ca; Wolf, Cr; Rane, A (January 1995). "Evidence for a role of cytochrome P450 2D6 and 3A4 in ethylmorphine metabolism". British Journal of Clinical Pharmacology. 39 (1): 77–80. doi:10.1111/j.1365-2125.1995.tb04413.x. ISSN 1365-2125. PMC 1364985. PMID 7756104.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.