Nimesulide
Nimesulide is a nonsteroidal anti-inflammatory drug (NSAID) with pain medication and fever reducing properties. Its approved indications are the treatment of acute pain, the symptomatic treatment of osteoarthritis, and primary dysmenorrhoea in adolescents and adults above 12 years old.
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth, rectal, topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | >97.5% |
| Metabolism | Hepatic |
| Elimination half-life | 1.8–4.7h |
| Excretion | Renal (50%), fecal (29%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.052.194 |
| Chemical and physical data | |
| Formula | C13H12N2O5S |
| Molar mass | 308.31 g·mol−1 |
| 3D model (JSmol) | |
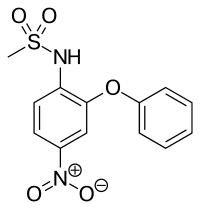
SMILES
| |
InChI
| |
| | |
Side effects may include liver problems.[1] It has a multifactorial mode of action and is characterized by a fast onset of action. It works by blocking the production of prostaglandins (a chemical associated with pain), thereby relieving pain and inflammation.
Medical uses
Nimesulide is not available in the United States, however is used in other countries for acute pain.[1] It may be used for pain, including period pains. Nimesulide is not recommended long-term, as for chronic conditions such as arthritis. This is due to its association with an increased risk of liver toxicity, including liver failure.[2]
Children
Less than 10 days of nimesulide does not appear to increase the risk of hypothermia, gastrointestinal bleeding, epigastric pain, vomiting, diarrhea, or transient, asymptomatic elevation of liver enzymes compared to ketoprofen, paracetamol, mefenamic acid, aspirin, or ibuprofen in children. However, data does not speak to populations less than 6 months old.[3]
Pregnancy and lactation
Women should use the drug with caution during lactation and nimesulide is contraindicated during pregnancy, and research suggest that it is also contraindicated in lactating women.[4]
Contraindications
It should be avoided by children under 12 and people with liver problems or flu-like symptoms.
Side effects
Nimesulide is similar in gastrointestinal safety to other NSAIDs. Due to concerns about the risk of liver toxicity, nimesulide has been withdrawn from market in several countries (Spain, Finland, Belgium, Ireland, and United States).[5] Liver problems have resulted in both deaths and the need for transplantation.[1] This may occur in as little as three days after starting the medication.[1]
Continuous use of nimesulide (more than 15 days) may cause the following side effects:
- Diarrhea
- Vomiting
- Skin rash
- Itchiness
- Dizziness
- Bitterness in mouth
Pharmacokinetics
Nimesulide is absorbed rapidly following oral administration.[6]
Nimesulide undergoes extensive biotransformation, mainly to 4-hydroxynimesulide (which also appears to be biologically active).[6]
Food, gender, and advanced age have negligible effects on nimesulide pharmacokinetics.[6]
Moderate chronic kidney disease does not necessitate dosage adjustment, while in patients with severe chronic kidney disease or liver disease, Nimesulide is contraindicated.[7]
Nimesulide has a relatively rapid onset of action, with meaningful reductions in pain and inflammation observed within 15 minutes from drug intake.[8][9]
The therapeutic effects of Nimesulide are the result of its complex mode of action, which targets a number of key mediators of the inflammatory process such as: COX-2 mediated prostaglandins, free radicals, proteolytic enzymes, and histamine.[8] Clinical evidence is available to support a particularly good profile in terms of gastrointestinal tolerability.[10]
History
It was launched in Italy for the first time as Aulin and Mesulid in 1985 and is available in more than 50 countries worldwide, including among others France, Portugal, Greece, Switzerland, Belgium, Russia, Thailand, and Brazil.[11] Nimesulide has never been filed for Food and Drug Administration (FDA) evaluation in the United States, where it is not marketed.
Availability
It is available in a variety of forms: tablets, powder for dissolution in water, suppositories, mouth dissolving tablets, and topical gel.
A recent evaluation from the European Medicines Agency (EMA) concluded that the overall benefit/risk profile of nimesulide is favourable and in line with that of the other NSAIDs such as diclofenac, ibuprofen, and naproxen.
Trade names
Nimesulide is available throughout the world as original product with the following trademarks: Sulide, Nimalox, Mesulid (Novartis, Brazil, Boehringer Ingelheim, Greece), Coxtal (Sanofi, China, Czech, Bulgaria), Sintalgin (Abbott, Brazil), Eskaflam (GSK, Brazil, Mexico), Octaprin, Nimside (Teva, Pakistan), Nise (Russia, Venezuela, Vietnam, Ukraine), Nilsid (Egypt); Aulin (Bulgaria, Czechia), Ainex, Drexel, Donulide, Edrigyl, Enetra, Heugan, Mesulid, Minapon, NeRelid, Nexen, Nidolon, Nilden (Mexico); Emsulide, Nimed, Nimedex, Nimesil (Czechia, Moldova), Nimulid (Trinidad & Tobago), Nimutab, Nimdase, Nimopen-MP Nise, Nisulid, Nodard Plus, Nicip, Nimcap, Nic-P, Nic-Spas (India); Mesulid, Novolid, Relmex (Ecuador); Remisid (Ukraine); Coxulid, Emulid, Frenag, Fuzo, Motival, Nimeksil, Nimelid, Nîmes, Nimesdin, Romasulid, Sulidin, Suljel, Thermo Sulidin (Turkey), Xilox (Hungary); Modact-IR (Pakistan); and ad Sulidene and Zolan for veterinary use. Many generic and copy-products also exist (Lusemin, Medicox, Nidol, Nimalox, Nimesil, Nimotas, Nimulid, Nizer, Sorini, Ventor, Vionim, Neolide, Willgo among others), new-aid (Syria), Nims, Nice, Nimulide (Nepal)
Society and culture
India
Several reports have been made of adverse drug reactions in India.[12][13][14][15] On February 12, 2011, Indian Express reported that the Union Ministry of Health and Family Welfare finally had decided to suspend the pediatric use of the analgesic, Nimesulide suspension.[16] From 10 March 2011 onward Nimesulide formulations are not indicated for human use in children below 12 years of age.[17]
On September 13, 2011 Madras High Court revoked a suspension on manufacture and sale of paediatric drugs nimesulide and phenylpropanolamine (PPA).[18]
EMA confirms the positive benefit/risk ratio
On September 21, 2007 the EMA released a press release on their review on the liver-related safety of nimesulide. The EMA has concluded that the benefits of these medicines outweigh their risks, but that there is a need to limit the duration of use to ensure that the risk of patients developing liver problems is kept to a minimum. Therefore, the EMA has limited the use of systemic formulations (tablets, solutions, suppositories) of nimesulide to 15 days.[19]
Irish Medicines Board
The Irish Medicines Board has decided to suspend Nimesulide from the Irish market and refer it to the EU Committee for Human Medicinal Products (CHMP) for a review of its benefit/risk profile. The decision is due to the reporting of six cases of potentially-related liver failures to the IMB by the National Liver Transplant Unit, St. Vincent's University Hospital. These cases occurred in the period from 1999 to 2006.[20]
Bribes
In May 2008, Italy's leading daily paper Corriere della Sera and other media outlets reported that a top-ranking official at Italy's medicines agency AIFA had been filmed by police while accepting bribes from employees of pharmaceutical companies.[21][22] The money allegedly was being paid to ensure that certain drugs would be spared scrutiny from the drugs watchdog. The investigation had started in 2005 following suspicions that some AIFA drug tests had been faked. Eight arrests were made. Nimesulide can be bought carrying a prescription from a physician that is kept as a receipt at the chemist shop, nominally allowing strong control over selling.
The original manufacturer of Nimesulide is Helsinn Healthcare SA, Switzerland, which acquired the rights for the drug in 1976. After the patent protection terminated, a number of other companies started production and marketing of Nimesulide.
References
- "Nimesulide". livertox.nih.gov. Archived from the original on 23 December 2017. Retrieved 22 December 2017.
- "Current status: European Commission final decision". Archived from the original on 19 October 2014. Retrieved 12 November 2014.
- Gupta, P; Sachdev, HP (June 2003). "Safety of oral use of nimesulide in children: systematic review of randomized controlled trials". Indian pediatrics. 40 (6): 518–31. PMID 12824661.
- "Nimesulide, Nimesulide Tablets, Ainex Nimesulide, Nimesulide Manufacturers". www.pharmaceutical-drug-manufacturers.com. Archived from the original on 2016-03-03.
- McNaughton, R.; Huet, G.; Shakir, S. (2014). "An investigation into drug products withdrawn from the EU market between 2002 and 2011 for safety reasons and the evidence used to support the decision-making". BMJ Open. 4 (1): e004221. doi:10.1136/bmjopen-2013-004221. PMC 3902466. PMID 24435895. Archived from the original on 2015-03-22.

- Bernareggi, Alberto (October 1998). "Clinical pharmacokinetics of nimesulide". Clinical Pharmacokinetics. 35 (4): 247–74. doi:10.2165/00003088-199835040-00001. PMID 9812177.
- Microsoft Word - opnh.P.Nimesulide .EMEA-CPMP-3086-03-en-Final.doc Archived 2007-06-11 at the Wayback Machine
- Rainsford KD (June 2006). "Nimesulide – a multifactorial approach to inflammation and pain: scientific and clinical consensus". Curr Med Res Opin. 22 (6): 1161–70. doi:10.1185/030079906X104849. PMID 16846549.
- Bianchi, M.; Broggini, M. (2003). "A randomised, double-blind, clinical trial comparing the efficacy of nimesulide, celecoxib and rofecoxib in osteoarthritis of the knee". Drugs. 63 Suppl 1: 37–46. doi:10.2165/00003495-200363001-00006. PMID 14506910.
- Laporte JR, Ibáñez L, Vidal X, Vendrell L, Leone R (2004). "Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents". Drug Saf. 27 (6): 411–20. doi:10.2165/00002018-200427060-00005. PMID 15144234.
- K.D. Rainsford (red.) (2005). Nimesulide – Actions and Uses. Bazylea, Boston, Berlin: Birkhäuser Verlag. p. 7. ISBN 978-3-7643-7068-8.
- Safety of nimesulide. CD ROM, Appropriate Use of Antipyretics / Analgesics in Children, Health Informatics, New Delhi, 2004.
- Rahman SZ, Khan RA (2004). "Is nimesulide safe in a cardiovascular-Compromised patient?". Indian J Pharmacol. 36: 252–3.
- Khan RA, Rahman SZ (2004). "A Case Report on Nimesulide and its Relation with Angina". J Pharmacovigilance Drug Safety. 1: 19–21.
- Khan RA, Rahman SZ (2004). "Nimesulide Induced Coronary Artery Insufficiency – A Case Report". J Pharmacovigilance Drug Safety. 1: 11–3.
- Thacker, Teena (12 February 2011). "Nimesulide for kids to be banned, finally". The Indian Express. Retrieved 2018-05-19.
- CDSCO website-wide gazette notification GSR 82(E) dated 10.03.2011.
- "Madras High Court Revokes Ban on Manufacture and Sale PPA - Adverse Effect - Medical Treatments". Scribd. Archived from the original on 2014-01-07.
- EMA press release on nimesulide September 2007 Archived 2009-07-18 at the Portuguese Web Archive
- "Error Page". www.imb.ie.
- «Mazzette per evitare i controlli sull'Aulin». Mario Pappagallo, Corriere della Sera, 23 May 2008 Archived May 25, 2008, at the Wayback Machine
- "Italian medicines agency officials arrested in corruption probe". Manufacturing Chemist. 22 May 2008. Retrieved 2018-05-19.