Selexipag
Selexipag (brand name Uptravi) is a drug developed by Actelion for the treatment of pulmonary arterial hypertension (PAH). Selexipag and its active metabolite, ACT-333679 (or MRE-269, the free carboxylic acid), are agonists of the prostacyclin receptor, which leads to vasodilation in the pulmonary circulation.[1]
 | |
| Names | |
|---|---|
| IUPAC name
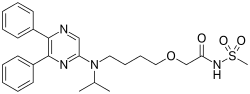
2-{4-[(5,6-diphenylpyrazin-2-yl)(propan-2-yl)amino]butoxy}-N-(methanesulfonyl)acetamide | |
| Other names
ACT-293987, NS-304 | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.237.916 |
| KEGG | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C26H32N4O4S |
| Molar mass | 496.6 g·mol−1 |
| Pharmacology | |
| B01AC27 (WHO) | |
| License data | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Contraindications
In Europe, use of selexipag together with strong inhibitors of the liver enzyme [CYP2C8], such as gemfibrozil, is contraindicated because it increases concentrations of selexipag twofold, and its active metabolite 11-fold, potentially leading to more adverse effects.[2]
Adverse effects
The adverse effects of selexipag are similar to those of intravenous prostacyclins used for pulmonary arterial hypertension. Common side effects include headache and jaw pain. An increased risk for hyperthyroidism has also been noted in people taking selexipag.
History
The US FDA granted selexipag Orphan Drug status for PAH.[3] It was approved by the US FDA on 22 December 2015.[3] The expected price for the drug in the US is $160,000 to $170,000 per patient before rebates.[4]
In Europe, the drug was approved in May 2016.[5]
See also
- Epoprostenol, another name for prostacyclin
- Analogues of prostacyclin:
References
- Sitbon, O.; Morrell, N. (2012). "Pathways in pulmonary arterial hypertension: The future is here". European Respiratory Review. 21 (126): 321–327. doi:10.1183/09059180.00004812. PMID 23204120.
- Information des Bundesamtes für Sicherheit im Gesundheitswesen zu Uptravi (in German), Österreichisches Bundesamt für Sicherheit im Gesundheitswesen, 2017-06-07
- New Drug Approved for Rare Lung Disorder. PPN. 23 Dec 2015 Has link to GRIPHON study results
- "Actelion sees Uptravi price of $160,000-170,000/patient". Reuters. 2016-01-05. Retrieved 2016-01-06.
- "Uptravi: Authorisation details". European Medicines Agency. 2016-05-12.