Macitentan
Macitentan (trade name Opsumit) is an endothelin receptor antagonist (ERA) approved for the treatment of pulmonary arterial hypertension (PAH).[1] The other two ERAs marketed as of 2014 are bosentan and ambrisentan.[1] Macitentan is a dual ERA, meaning that it acts as an antagonist of two endothelin (ET) receptor subtypes, ETA and ETB.[1] However, macitentan has a 50-fold increased selectivity for the ETA subtype compared to the ETB subtype.[2] The drug received approval from the U.S. Food and Drug Administration (FDA) on October 13, 2013.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Opsumit |
| Other names | ACT-064992 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hydrolysis, oxidation (CYP3A4) |
| Excretion | 2/3 urine, 1/3 faeces |
| Identifiers | |
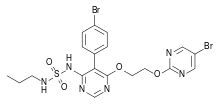
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H20Br2N6O4S |
| Molar mass | 588.28 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Adverse effects
The FDA has issued a black box warning for macitentan for embryo-fetal toxicity. It is only available to females in the U.S through a risk evaluation and mitigation strategy (REMS) program.[4]
Mechanism of action
Endothelin and endothelin receptors
Endothelin (ET) is an extremely potent blood vessel constricting substance that is secreted by endothelial cells.[5] In the lungs, the most common ET form released is ET-1.[5] ET-1 release can occur through both constitutive and non-constitutive pathways.[5] Upon release, ET-1 can bind to the ET receptors that are expressed on arterial smooth muscle cells and fibroblasts in the lungs.[5] ET receptors are G protein coupled receptors and, when activated, lead to an increase in intracellular calcium levels via the Gαq signaling pathway.[5] The rise in intracellular calcium leads to contraction of the arterial smooth muscle, as well as vascular remodelling due to cell proliferation.[5] Prolonged constriction and fibrosis are factors in the pathogenesis of PAH.[1]
Role of macitentan
Macitentan blocks the ET1-dependent rise in intracellular calcium by inhibiting the binding of ET-1 to ET receptors. Blocking of the ETA receptor subtype seems to be of more importance in the treatment of PAH than blocking of ETB, likely because there are higher numbers of ETA receptors than ETB receptors in pulmonary arterial smooth muscle cells.[5]
Pharmacokinetics
Macitentan is taken as a 10 mg oral dose once a day.[1] Its half-life in humans is about 16 hours and steady state is reached by the third day of administration.[6] It is absorbed slowly into the plasma.[7] Macitentan dealkylates into the active metabolite ACT-132577, which reaches its peak plasma concentration about 30 hours after the first dose is administered, and has a half-life of approximately 48 hours.[7] Although ACT-132577 has a lower affinity for the ET receptors than its parent compound,[2] It maintains higher plasma concentrations than macitentan.[7] Both compounds can be excreted from the body through the urine or feces.[6]
Co-administration of ciclosporin has only a slight effect on the concentrations of macitentan and its active metabolite, while rifampicin decreases the area under the curve (AUC) of the drug's blood plasma concentration by 79%, and ketoconazole approximately doubles it. This corresponds to the finding that macitentan is mainly metabolised via the liver enzyme CYP3A4.[8]
Experimental pharmacokinetics
Macitentan has slow association kinetics.[5] Its potency increases 6.3-fold when it is pre-incubated with pulmonary arterial smooth muscle cells for 120 minutes compared to 10 minutes with pulmonary arterial smooth muscle cells.[5] Macitentan also has a high receptor occupancy half-life (approximately 17 minutes) compared to bosentan (approximately 70 seconds) and ambrisentan (approximately 40 seconds).[5] This increased receptor occupancy half-life allows macitentan to act as a non-competitive antagonist of ET receptors.[5] Bosentan and ambrisentan are both competitive antagonists.[5]
References
- Hong IS, Coe HV, Catanzaro LM (April 2014). "Macitentan for the treatment of pulmonary arterial hypertension". The Annals of Pharmacotherapy. 48 (4): 538–47. doi:10.1177/1060028013518900. PMID 24458948.
- Iglarz M, Binkert C, Morrison K, Fischli W, Gatfield J, Treiber A, et al. (December 2008). "Pharmacology of macitentan, an orally active tissue-targeting dual endothelin receptor antagonist". The Journal of Pharmacology and Experimental Therapeutics. 327 (3): 736–45. doi:10.1124/jpet.108.142976. PMID 18780830.
- "Actelion receives us fda approval of Opsumit (macitentan) for the treatment of pulmonary arterial hypertension". Actelion. Archived from the original on 2013-10-23. Retrieved 22 October 2013.
- Opsumit [package insert] Whippany, NJ: Bayer HealthCare Pharmaceuticals 2017
- Gatfield J, Mueller Grandjean C, Sasse T, Clozel M, Nayler O (2012). "Slow receptor dissociation kinetics differentiate macitentan from other endothelin receptor antagonists in pulmonary arterial smooth muscle cells". PLOS ONE. 7 (10): e47662. Bibcode:2012PLoSO...747662G. doi:10.1371/journal.pone.0047662. PMC 3471877. PMID 23077657.
- Bruderer S, Hopfgartner G, Seiberling M, Wank J, Sidharta PN, Treiber A, Dingemanse J (September 2012). "Absorption, distribution, metabolism, and excretion of macitentan, a dual endothelin receptor antagonist, in humans". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 42 (9): 901–10. doi:10.3109/00498254.2012.664665. PMID 22458347.
- Sidharta PN, van Giersbergen PL, Halabi A, Dingemanse J (October 2011). "Macitentan: entry-into-humans study with a new endothelin receptor antagonist". European Journal of Clinical Pharmacology. 67 (10): 977–84. doi:10.1007/s00228-011-1043-2. PMC 3169777. PMID 21541781.
- Bruderer S, Aänismaa P, Homery MC, Häusler S, Landskroner K, Sidharta PN, et al. (March 2012). "Effect of cyclosporine and rifampin on the pharmacokinetics of macitentan, a tissue-targeting dual endothelin receptor antagonist". The AAPS Journal. 14 (1): 68–78. doi:10.1208/s12248-011-9316-3. PMC 3282010. PMID 22189899.