Seltorexant
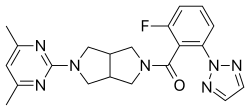
Seltorexant (former developmental code names MIN-202, JNJ-42847922, JNJ-922) is a selective, small-molecule antagonist of the OX2 receptor that is under development by Minerva Neurosciences and Johnson & Johnson's Janssen Pharmaceutica for the treatment of insomnia and major depressive disorder (MDD).[1][2][3] As of December 2015, it is in phase II clinical trials for both insomnia and MDD.[2][4][5]
 | |
| Clinical data | |
|---|---|
| Other names | MIN-202; JNJ-42847922; JNJ-922 |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| Chemical and physical data | |
| Formula | C21H22FN7O |
| Molar mass | 407.443 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Christopher, John A (2014). "Small-molecule antagonists of the orexin receptors". Pharmaceutical Patent Analyst. 3 (6): 625–638. doi:10.4155/ppa.14.46. ISSN 2046-8954. PMID 25489915.
- Zisapel, Nava (2014). "Current Phase II investigational therapies for insomnia". Expert Opinion on Investigational Drugs. 24 (3): 1–11. doi:10.1517/13543784.2015.987340. ISSN 1354-3784. PMID 25423562.
- Cristoph Boss; Catherine Ross (2015). "Recent Trends in Orexin Research – 2010 to 2015". Bioorganic & Medicinal Chemistry Letters. 25 (15): 2875–2887. doi:10.1016/j.bmcl.2015.05.012. PMID 26045032.
- "JNJ 42847922". AdisInsight. Retrieved 2015-05-19.
- Medicines in Development for Mental Health (PDF) (Report). Pharmaceutical Research and Manufacturers of America. 2014. Retrieved 2015-05-19.
Further reading
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.