Suvorexant
Suvorexant, sold under the trade name Belsomra, is a medication for the treatment of insomnia.[1] It is effective for insomnia, at least for four weeks and as compared to a placebo.[2]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Belsomra |
| Other names | MK-4305 |
| AHFS/Drugs.com | belsomra |
| MedlinePlus | a614046 |
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 82% (at 10 mg) |
| Protein binding | >99% |
| Metabolism | hepatic, CYP3A, CYP2C19 |
| Elimination half-life | ~12 hours |
| Excretion | Feces (66%), urine (23%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.546 |
| Chemical and physical data | |
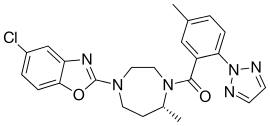
| Formula | C23H23ClN6O2 |
| Molar mass | 450.92 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Suvorexant is a selective, dual orexin receptor antagonist (DORA) made by Merck & Co. It was approved for sale by the U.S. Food and Drug Administration (FDA) on August 13, 2014.[3] The U.S. Drug Enforcement Administration (DEA) has placed it on the list of schedule IV controlled substances,[4] as it may lead to limited physical dependence or psychological dependence relative to the drugs or other substances in schedule III. The potential for psychological dependence is similar to that of zolpidem.[5]
The drug was initially released November 2014 in Japan[6], then later reached the United States in February 2015[7] and Australia in November 2016.
Medical uses
Suvorexant is used for the treatment of insomnia, characterized by difficulties with sleep onset and/or sleep maintenance.[8]
It is unclear how the medication compares to others used for insomnia as no comparisons have been done.[2] It is also unclear if this medication is safe among people with a history of addiction, as they were excluded from the clinical trials of suvorexant.[2]
Side effects
The most common complaint about the drug is from users who report that it did not help them to sleep.[9] Some people reported that the drug caused a sleep disturbance such as a nightmare, sleep terror, or abnormal dream.[9][10] Others reported that the drug caused them to be more awake.[9]
Issues include sleepiness the next day and issues with driving.[10] Other concerns include thoughts of suicide.[10]
Contraindications
This drug is not recommended in people with liver impairment.[11] Suvorexant pregnancy category is currently classified as Category C.[12] Based on animal testing, this medication may cause fetal harm during pregnancy and should only be given in pregnancy if the potential benefit justifies the potential harm to the fetus. Evidence is inconclusive about whether using this medication while breastfeeding puts the infant at risk of harm.[11]
Suvorexant is contraindicated in people diagnosed with narcolepsy.[12]
Interactions
Suvorexant is not recommended if people are also taking medications that strongly inhibit the liver enzyme CYP3A like itraconazole, lopinavir/ritonavir, clarithromycin, ritonavir, ketoconazole, indinavir/ritonavir, or conivaptan.[11][13] If suvorexant is used with a medication that moderately inhibits the liver enzyme CYP3A, like verapamil, erythromycin, diltiazem, or dronedarone, it is recommended that the dose of suvorexant be adjusted.[11][13]
Mechanism of action
Suvorexant exerts its therapeutic effect in insomnia through antagonism of orexin receptors. The orexin neuropeptide signaling system is a central promoter of wakefulness. Blocking the binding of wake-promoting neuropeptides orexin A and orexin B to receptors orexin receptor type 1 (OX1) and orexin receptor type 2 (OX2) is thought to suppress wake drive.[8] Animal studies report the binding affinities for OX1 (0.55 nM) and OX2 (0.35 nM).[14]
Pharmacokinetics
The bioavailability of suvorexant is at 82%. It is highly protein-bound. Food delays the time to max concentration. The primary route of elimination is through the feces, with approximately 66% of radiolabeled dose recovered in the feces compared to 23% in the urine. The elimination half-life is reported to be 12 hours.[12]
Abuse liability
According to the U.S. Drug Enforcement Administration (DEA), suvorexant produces similar reinforcing effects to those of zolpidem in humans and thus may have a similar abuse liability.[15] As such, suvorexant has been designated a schedule IV controlled substance in the U.S. under the Controlled Substances Act.[15]
See also
References
- Baxter, C. A.; Cleator, E.; Brands, K. M. J.; Edwards, J. S.; Reamer, R. A.; Sheen, F. J.; Stewart, G. W.; Strotman, N. A.; Wallace, D. J. (2011). "The First Large-Scale Synthesis of MK-4305: A Dual Orexin Receptor Antagonist for the Treatment of Sleep Disorder". Organic Process Research & Development. 15 (2): 367–375. doi:10.1021/op1002853.
- Patel, K. V; Aspesi, A. V; Evoy, K. E (Feb 9, 2015). "Suvorexant: A Dual Orexin Receptor Antagonist for the Treatment of Sleep Onset and Sleep Maintenance Insomnia". Ann Pharmacother. 49 (4): 477–483. doi:10.1177/1060028015570467. PMID 25667197.
- https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm409950.htm
- "Schedules of Controlled Substances: Placement of Suvorexant into Schedule IV". federalregister.gov. Federal Register. February 13, 2014. Retrieved August 10, 2016.
A Proposed Rule by the Drug Enforcement Administration on 02/13/2014
- "Rules - 2014 - Final Rule: Placement of Suvorexant into Schedule IV". www.deadiversion.usdoj.gov. Retrieved 2016-04-03.
- "New hypnotic drug without addiction to be released in Japan first".
- "Merck's Insomnia Medicine Belsomra C-IV Now Available in US". www.sleepreviewmag.com. Sleep Review. Retrieved 9 September 2015.
- "Highlights of prescribing information" (PDF).
- Carr, Teresa (5 February 2016). "FDA Fields Complaints on Sleeping Pill Suvorexant". Consumer Reports.
- Jacobson, LH; Callander, GE; Hoyer, D (Nov 2014). "Suvorexant for the treatment of insomnia". Expert Review of Clinical Pharmacology. 7 (6): 711–30. doi:10.1586/17512433.2014.966813. PMID 25318834.
- Label: BELSOMRA- Suvorexant Tablet, Film Coated"Label: BELSOMRA- Suvorexant Tablet, Film Coated." DailyMed. Merck Sharp & Dohme Corp. & the U.S. National Library of Medicine, 01 Aug. 2014. Web. 29 Oct. 2014.
- Product Information: BELSOMRA(R) oral tablets, suvorexant oral tablets. Merck Sharp & Dohme Corp. (per manufacturer), Whitehouse Station, NJ, 2014.
- "U.S. Food and Drug Administration." Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. U.S. Food and Drug Administration, 27 Oct. 2014. Web. 30 Oct. 2014.
- "Suvorexant Advisory Committee Meeting Briefing Document" (PDF). May 22, 2013. Retrieved Feb 7, 2015.
- Drug Enforcement Administration, Department of Justice (2014). "Schedules of controlled substances: placement of suvorexant into Schedule IV. Final rule" (PDF). Fed Regist. 79 (167): 51243–7. PMID 25167596.
External links
- Belsomra (official website)
- "The Big Sleep" (The New Yorker, 2013)—detailed article on development and approval process