Darusentan
Darusentan (LU-135252; HMR-4005) is an endothelin receptor antagonist. Gilead Colorado, a subsidiary of Gilead Sciences,[1] under license from Abbott Laboratories, is developing darusentan for the potential treatment of uncontrolled hypertension.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 12.5 hours |
| Identifiers | |
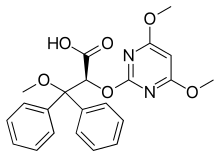
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.126.841 |
| Chemical and physical data | |
| Formula | C22H22N2O6 |
| Molar mass | 410.42 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
In June 2003, Myogen licensed the compound from Abbott for its application in the cancer field.[2]
In May 2007, a randomized, double-blind, active control, parallel assignment, safety and efficacy phase III trial was initiated in subjects who had completed the maintenance period of the DAR-312 study.
See also
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.