Almorexant
Almorexant (INN; development code ACT-078573) is an orexin antagonist, functioning as a competitive receptor antagonist of the OX1 and OX2 orexin receptors, which was being developed by the pharmaceutical companies Actelion and GSK for the treatment of insomnia. Development of the drug was abandoned in January 2011 due to undisclosed issues pertaining to almorexant's safety profile.[1]
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| ATC code |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
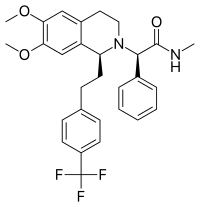
| Formula | C29H31F3N2O3 |
| Molar mass | 512.573 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Development
Originally developed by Actelion, from 2007 almorexant was being reported as a potential blockbuster drug, as its novel mechanism of action (orexin receptor antagonism) was thought to produce better quality sleep and fewer side effects than the traditional benzodiazepines and Z-drugs which dominated the multibillion-dollar insomnia medication market.[2]
In 2008, GlaxoSmithKline bought the development and marketing rights for almorexant from Actelion for an initial payment of $147 million.[3] The deal would have been worth an estimated $3.2 billion if the drug had successfully completed clinical development and obtained FDA approval.[4] GSK and Actelion continued to develop the drug together, and completed a Phase III clinical trial in November 2009.[5]
However, in January 2011 Actelion and GSK announced they were abandoning the development of almorexant because of its side effect profile.[1][6]
Mechanism of action
Almorexant is a competitive, dual OX1 and OX2 receptor antagonist and selectively inhibits the functional consequences of OX1 and OX2 receptor activation, such as intracellular Ca2+ mobilization.
See also
References
- GSK and Actelion discontinue clinical development of almorexant Archived 2011-07-04 at the Wayback Machine - GSK press release, 28 Jan 2011
- Sleeping Beautifully - CBS Business Network 24 Sep 2007
- Actelion Sells Glaxo Almorexant Sleep Medicine Rights - Bloomberg, 14 July 2008
- Actelion's top dollar deal leaves doubts, and little on the horizon - EP Vantage, 14 July 2008
- Almorexant in Adult Subjects With Chronic Primary Insomnia (RESTORA 1). ClinicalTrials.gov (February 3, 2010). Retrieved on May 6, 2010.
- Actelion and GSK Discontinue Clinical Development of Almorexant Archived 2011-03-03 at the Wayback Machine - Actelion press release, 28 Jan 2011