AdipoRon
AdipoRon is a selective, orally active, synthetic small-molecule agonist of the adiponectin receptor 1 (AdipoR1) and adiponectin receptor 2 (AdipoR2) (Kd = 1.8 μM and 3.1 μM, respectively).[1][2] It activates AMPK and PPARα signaling and ameliorates insulin resistance, dyslipidemia, and glucose intolerance in db/db mice (an animal model for type II diabetes and obesity).[1][2] Moreover, AdipoRon has been found to extend the lifespans of db/db mice fed a high-fat diet, as well as improve exercise endurance.[1][2][3] The compound was discovered by Japanese researchers in 2013 via screening of a compound library, and is the first orally active, small-molecule agonist of the adiponectin receptors to be identified.[1][2]
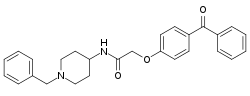 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C27H28N2O3 |
| Molar mass | 428.52282 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Adiponectin receptor agonists such as AdipoRon have attracted interest as potential therapies for obesity, diabetes, cardiovascular disease, non-alcoholic fatty liver disease, and a panoply of other conditions.[1][2] In addition, adiponectin has recently been elucidated to mediate the antidepressant, anxiolytic, and neurogenic effects of physical exercise.[4][5][6] Dysregulation of adiponectin expression has also been implicated in the pathology of mood disorders, anxiety disorders, eating disorders, neurodegenerative disorders, and various other neuropsychiatric disorders.[7] Also, it has been determined that exercise improves insulin resistance via activation of AdipoR1.[8] As such, adiponectin receptor agonists are a highly interesting therapeutic target for a variety of different conditions.[1][2][6][7] Moreover, it has been suggested they could potentially be used as a substitute for exercise to achieve similar physical and mental health benefits.[1][2][6][9]
In 2016, the University of Tokyo announced it was launching an investigation into anonymously made claims of fabricated and falsified data on the identification of AdipoR1, AdipoR2 and AdipoRon.[10]
References
- Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, Ogata H, Tokuyama K, Ueki K, Nagano T, Tanaka A, Yokoyama S, Kadowaki T (2013). "A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity". Nature. 503 (7477): 493–9. doi:10.1038/nature12656. PMID 24172895.
- Holland, W. L.; Scherer, P. E. (2013). "Ronning After the Adiponectin Receptors". Science. 342 (6165): 1460–1461. doi:10.1126/science.1249077. ISSN 0036-8075. PMC 4084614. PMID 24357309.
- Okada-Iwabu M, Iwabu M, Ueki K, Yamauchi T, Kadowaki T (2015). "Perspective of Small-Molecule AdipoR Agonist for Type 2 Diabetes and Short Life in Obesity". Diabetes Metab J. 39 (5): 363–72. doi:10.4093/dmj.2015.39.5.363. PMC 4641965. PMID 26566493.
- Yau SY, Li A, Hoo RL, Ching YP, Christie BR, Lee TM, Xu A, So KF (2014). "Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin". Proc. Natl. Acad. Sci. U.S.A. 111 (44): 15810–5. doi:10.1073/pnas.1415219111. PMC 4226125. PMID 25331877.
- Nicolas S, Veyssière J, Gandin C, Zsürger N, Pietri M, Heurteaux C, Glaichenhaus N, Petit-Paitel A, Chabry J (2015). "Neurogenesis-independent antidepressant-like effects of enriched environment is dependent on adiponectin". Psychoneuroendocrinology. 57: 72–83. doi:10.1016/j.psyneuen.2015.03.017. PMID 25889841.
- Li A, Yau SY, Machado S, Yuan TF, So KF (2015). "Adult neurogenic and antidepressant effects of adiponectin: a potential replacement for exercise?". CNS Neurol Disord Drug Targets. 14 (9): 1129–1144. doi:10.2174/1871527315666151111125533. PMID 26556072.
- Wędrychowicz, Andrzej (2014). "Peptides from adipose tissue in mental disorders". World Journal of Psychiatry. 4 (4): 103–111. doi:10.5498/wjp.v4.i4.103. ISSN 2220-3206. PMC 4274582. PMID 25540725.
- Cho JK, Kim S, Hong HR, Yoon JH, Kang H (2015). "Exercise Training Improves Whole Body Insulin Resistance via Adiponectin Receptor 1". Int J Sports Med. 36 (13): e24–e30. doi:10.1055/s-0035-1559715. PMID 26528942.
- Yau SY, Li A, Xu A, So KF (2015). "Fat cell-secreted adiponectin mediates physical exercise-induced hippocampal neurogenesis: an alternative anti-depressive treatment?". Neural Regen Res. 10 (1): 7–9. doi:10.4103/1673-5374.150637. PMC 4357120. PMID 25788905.
- University of Tokyo to investigate data manipulation charges against six prominent research groups ScienceInsider, Dennis Normile, Sep 20, 2016