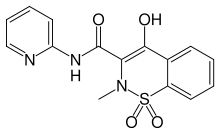
Oxicam
Oxicam is a class of nonsteroidal anti-inflammatory drugs (NSAIDs) that bind closely to plasma proteins.[1] Most oxicams are unselective inhibitors of the cyclooxygenase (COX) enzymes. The exception is meloxicam with a slight (10:1) preference for COX-2, which, however, is only clinically relevant at low doses.[2]
Examples include:
Chemistry
The physico-chemical characteristics of these molecules vary greatly depending upon the environment.[3]
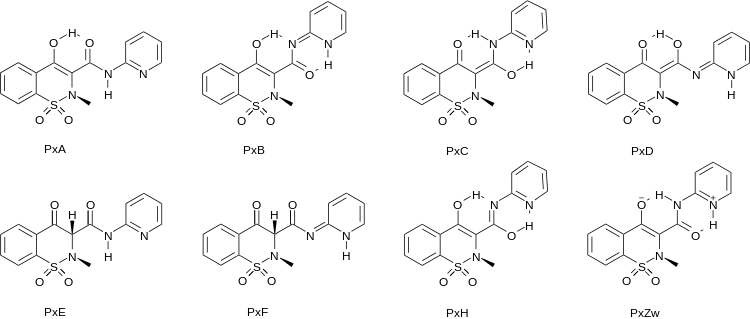
In contrast to most other NSAIDs, oxicams are not carboxylic acids. They are tautomeric and can exist as a number of tautomers (keto-enol tautomerism), here exemplified by piroxicam:[4]
Side Effects
The oxicams are associated with drug-related erythema multiforme (EM), Stevens-Johnson syndrome, and toxic epidermal necrolysis (TEN). This association is one of the reasons Oxicams are not regularly prescribed.
References
- Olkkola KT, Brunetto AV, Mattila MJ (1994). "Pharmacokinetics of oxicam nonsteroidal anti-inflammatory agents". Clinical Pharmacokinetics. 26 (2): 107–20. doi:10.2165/00003088-199426020-00004. PMID 8162655.
- Mutschler, Ernst; Gerd Geisslinger; Heyo K. Kroemer; Monika Schäfer-Korting (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 233. ISBN 3-8047-1763-2.
- Banerjee R, Chakraborty H, Sarkar M (2003). "Photophysical studies of oxicam group of NSAIDs: piroxicam, meloxicam and tenoxicam". Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy. 59 (6): 1213–22. doi:10.1016/S1386-1425(02)00300-1. PMID 12659890.
- Ivanova D, Deneva V, Nedeltcheva D, Kamounah FS, Gergov G, Hansen PE, Kawauchi S, Antonov L (2015). "Tautomeric transformations of piroxicam in solution: a combined experimental and theoretical study". RSC Advances. 5: 31852–31860. doi:10.1039/c5ra03653d.